Hepatoblastoma: current knowledge and promises from preclinical studies
Introduction
Hepatoblastoma (HB) is the most frequent liver malignancy in childhood. In most cases, HB is sporadic, although it may also be associated with Beckwith-Wiedemann syndrome, hemihypertrophy, premature birth, low birth weight, and familial adenomatous polyposis coli. The survival rate at 5 years of children affected by metastatic HB has steadily increased from 27% reported in the 1990s to the current 79%. This impressive result comes from the achievements of four trial groups: the International Childhood Tumor Strategy Group (SIOPEL), the Children Oncology Group (COG), the German Society for Pediatric Oncology and Haematology (GPOH), and the Japanese Study Group for Pediatric Liver Tumors (JPLT). These groups and their coordination organism, the Children’s Hepatic tumors International Collaboration (CHIC) consortium, established the standard of risk stratification and the treatment strategy of this rare tumor. Nowadays, the widely accepted risk stratification, is based on the radiological evaluation of the pre-therapy extent of the tumor (PRETEXT), the age of the patients, the serum levels of alpha-fetoprotein, the presence of metastatic disease, and the PRETEXT annotation factors (i.e., vascular involvement, extrahepatic contiguous extension, multifocality, rupture of the tumor at diagnosis). The PRETEXT staging system, initially proposed by the SIOPEL group, is based on the division of the liver into 4 sectors (1). The left sector consists of Couinaud segments 2 and 3, the medial segment is the Couinaud segment 4, the anterior sector of the right lobe consists of segments 5 and 8, the posterior sector of the right includes segments 6 and 7. PRETEXT I refers to patients where the tumor burden is limited to one sector. PRETEXT II indicates two sectors free of tumour; PRETEXT 3 indicates one sector free of tumor and in PRETEXT IV the tumor occupies all sectors. According to the unified analysis from the CHIC consortium, patients at high risk are: (I) PRETEXT I patients with positive annotation factors and age >8 years; (II) PRETEXT II and PRETEXT III patients with alpha-fetoprotein lower than 100 ng/mL, age >8 years; (III) PRETEXT IV patients when their age is >3 years and alpha-fetoprotein is lower than 100 ng/mL. Metastases represent an independent risk factor in all groups (2). Patients at intermediate risk are those with PRETEXT I, positive annotation factors and age >8 years; those with PRETEXT II, alpha feto-protein >100 ng/mL and positive annotation factors; those with PRETEXT III, alpha feto-protein between 100 and 1,000 ng/mL and positive annotation factors; those with PRETEXT IV and alpha feto-protein <100 ng/mL (2).
At the histopathological level, the main features of HB encompass the different stages of liver development, including epithelial and mixed epithelial and mesenchymal forms. Epithelial tumors include pure fetal with different grades of mitotic activity, embryonal, pleomorphic, macro trabecular, small cell undifferentiated with or without expression of INI1, cholangioblastic and mixed subtypes. The major HB subtypes are shown in Figure 1.
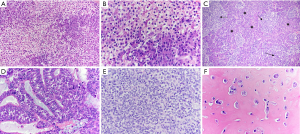
Although not yet included into the risk staging of the multivariate unified CHIC analysis, tumor histology also plays a role in patients’ outcome. In particular, the pure fetal subtype with low mitotic activity is associated with a better prognosis, whereas the small-cell undifferentiated subtype has the worst outcome. In addition, histology identifies the transitional liver cell tumor subtype, where both the features of HB and those of hepatocellular carcinoma (HCC) are recognized (3,4).
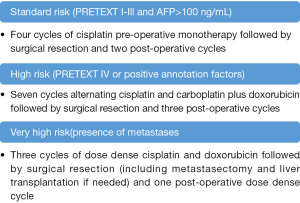
According to the SIOPEL guidelines, the current therapeutic strategy of patients with HB is based on the grade of risk. Patients at low risk and with the histological features of fetal subtype and low mitotic activity are cured by the surgical removal of the tumor, and do not need chemotherapy. In all other patients, neo-adjuvant chemotherapy is recommended. The pre-operative administration of cisplatin alone or combined with doxorubicin represents the mainstay treatment of HB (5). In patients at standard risk (PRETEXT I to III without metastases and annotation factors), cisplatin monotherapy is usually effective as neo-adjuvant treatment. Dose dense cisplatin and doxorubicin followed by surgery and post-operative chemotherapy is recommended for patients with metastases (6). These SIOPEL guidelines are reported in Figure 2. At variance, the COG group recommends upfront resection in all resectable patients and post-operative chemotherapy consisting of cisplatin, fluorouracil and vincristine. The rationale of this strategy is the de-intensification of post-operative chemotherapy (7). The answer to these controversies will be solved by the ongoing Paediatric Hepatic International Tumor Trial (PHITT). This trial also stems from the collaboration of the major groups involved in the treatment of HB and aims to optimize the strategy of therapy. Among others, the objectives of this study are: (I) to evaluate if the treatment of low risk HB can be reduced; (II) to compare different treatment regimens for intermediate risk HB; (III) to compare different post-induction treatment for high risk HB (8). Despite these indisputable achievements, several needs remain unmet, including the side-effects of chemotherapy, and the management of refractory cases. Well known side-effects of cisplatin are ototoxicity, neurotoxicity and nephrotoxicity. Among the patients treated with dose intense cisplatin, a Brock grade 3 ototoxicity was documented in 20%, and a Brock grade 4 in 7% of the cases (6). In addition, the limiting dose cardiotoxicity of doxorubicin hampers its potential efficacy. In patients refractory to cisplatin and doxorubicin the outcome remains poor, and the mechanism of drug resistance are still unclear (9).
Within this framework, new insights into the biology of HB are needed. In this review, we will focus on the following questions:
- Which are the driver genes of HB?
- Are these genes druggable?
- What is the role of immunotherapy?
- What is the relevance of in vivo models?
Driver genes of HB
In 1999 Koch et al. (10) reported for the first time that sporadic HB is the tumor with the highest in-frame mutation frequency of the CTNNB1 gene, encoding for β-catenin, the main transducer in the canonical WNT pathway (11). The WNT/β-catenin cascade has a key role in liver development, regeneration and metabolic zonation. When the WNT signalling is not activated, β-catenin is bound to a degradation complex consisting of Axin, APC, GSK3 and CK, and then is phosphorylated at specific serine and threonine residues in exon 3 and degraded by the ubiquitin proteasome pathway. When the WNT pathway is activated, β-catenin is stabilized and translocates into the nucleus, where it interacts with the T cell factor/lymphoid enhancement factor (TCF/LEF) family of transcription factors. Interactions with distinct transcription factors leads to the expression of different genes and functions. A similar scenario occurs when mutations of the exon 3 of the gene encoding for β-catenin take place. In patients with HB, the interacting transcription factor is TCF4 and target genes include, among others, c-MYC, Cyclin D1, EGFR, and glutamine synthetase (12).
Target genes of the dysregulated WNT/β catenin signaling are differently expressed in patients with distinct histological subtypes and clinical risk. Several molecular signatures of HB, based on gene expression have been proposed. For instance, Cairo et al. (13) reported a 16 gene-signature that differentiates standard-risk and high-risk patients. Tumor aggressiveness was associated with hepatic stem-like phenotypes and MYC upregulation. Overexpressed genes were MYCN, BIRC5, NPM1, HDAC2, TACSTD1, GJA1, and SUZ12. By contrast, standard-risk tumors were associated with the overexpression of GLUL, RHBG, CYP2E1, and CYP1A1. Sumazin et al. (14) analyzed 88 pre-treatment tumors and identified three distinct molecular clusters characterized by high, intermediate and low risk, according to the differential expression of hepatic progenitor cell markers and metabolic pathways. In particular, NFE2L2, HMGA2, SALL4 and AFP genes were strongly expressed and associated with the downregulation of let-7 and HNF1A in the most aggressive tumors. Hooks et al. (15) reported a simplified 4-gene signature, consisting of the differential expression of HSD17B6, ITGA6, TOP2A, and VIM. This molecular signature identifies one group of patients at low risk, and two subgroups at high risk. Further analysis of gene expression within the subgroups at high risk showed that epithelial-mesenchymal transition features and Fanconi anemia pathway were mutually expressed.
Immuno-histochemical phenotypes also contribute to the characterization of HB. Small-cell undifferentiated HBs are divided into two groups of different prognoses according to the expression of INI1, negative HBs behaving as rhabdoid tumors (16). Markers of stemness, such as EpCam, CK19, and AFP distinguished HB arising from stem cells from more mature types of the tumor (13).
Given the rarity of HB, the molecular and immunohistochemical biomarkers have not been validated in larger cohort of patients. The incorporation of the biological data into the clinical practice is one of the aims of the ongoing PHITT. The trial is collecting and characterizing the specimens of all recruited patients. Biological testing includes targeted sequencing, a next-generation sequencing mutation panel, a whole genome scanning SNP array platform, and histochemical analysis (8).
Crosstalk between signaling pathways
Similar to other solid tumors of childhood, HB is characterized by a low rate of mutated genes (17). When whole genome sequencing was performed, it appeared that the median rate of mutations is 3.9 per tumor (range, 0–24 mutations) (14). As expected, mutations increase with age. Besides CTNNB1, other mutated genes include NFE2L2, TERT promoter, APC, MLL2, ARID1A, SPOP, KLHL22, TRPC4AP, and RNF169 (18,19), but the number of tumors harboring these mutations is relatively low. It is therefore undisputable that CTNNB1 is the driver gene of sporadic HB. It is of interest, however, that the over-expression of full-length point mutant or deletion mutant β-catenin in mouse hepatocytes is insufficient for oncogenesis. Apart from the documented MYC activation, it has been hypothesized that other signaling pathways interact with WNT/β-catenin. Among these, activation of the Notch and Hedgehog pathways is documented by the upregulation of DLK and HES1 and GLI1 and PTCH1 genes, respectively (20,21). The interplay between β-catenin and YAP pathways may also play a role in the development of HB. In accordance with this hypothesis, immunohistochemistry showed the co-localization of β-catenin and YAP1 within the nuclei of 89% of 92 tested tumor specimens. In addition, the hydrodynamic expression of YAP1/β-catenin into the mouse liver resulted in the development of aggressive tumors with the histological features of HB (22).
Is the β-catenin pathway druggable?
The majority of the inhibitors of the canonical WNT/β catenin pathway are investigational molecules that target distinct steps of the WNT signaling. These agents include monoclonal antibodies directed against WNT ligands and WNT receptors, antagonists of porcupine, stabilizers of the β-catenin degradation complex, and, downstream of activated β-catenin, suppressors of the interaction of β-catenin with co-activator cyclic AMP response binding protein (23,24).
The inhibition of the cross talk between WNT/β-catenin pathway and other signaling pathways represents an active area of research in this field. Investigational inhibitors of Notch, such as PF-03084014, decrease the levels of activated β-catenin (25). The analysis of the signaling in the Yap/β-catenin mouse model showed that YAP induces the expression of the amino acid transporter SLC38A1 leading to mTOR activation. Targeting mTOR pathway or amino acid transporters may therefore represent a new therapeutic strategy (26). Furthermore, since β-catenin cooperates with Yap to induce HB development and suppression of Yap reduces β-catenin levels as well as β-catenin dependent growth and transforming activity (12,22), targeting Yap might be an intriguing alternative therapeutic approach to suppress/limit the WNT/β-catenin pathway in this tumor type. It is also of note that three components of the Fanconi anemia pathway (FANC1, FANC2D and BRCA1) were upregulated in patients resistant to doxorubicin. This pathway may be targeted by proteasome inhibitors such as bortezomib (15). Accordingly, the ClinicalTrials.Gov site (27) currently registers fifteen phase I/II studies whose aim is to evaluate the effect of several inhibitors such as multi-kinase inhibitors targeting CDK4/6, MAPK/ERK, ALK, and/or mTOR pathways.
What is the role of immunotherapy in the management of HB?
In the majority of patients, tumor infiltrating cells are scarce. This finding is in keeping with the low rate of mutations, the age of the patients, and the absence of co-morbid conditions. However, in APC related HBs, cisplatin leads to a massive increase of infiltrating cells organized in secondary and tertiary lymphoid structures (28). These cells consist of CD3 and CD8 positive lymphocytes and dendritic cells and are in direct contact with the tumor cells. It has been hypothesized that, in this case, chemotherapy leads to immunogenic cell death. Tumor cells damaged by cisplatin emit adjuvant signals recruiting the effectors of the adaptive response (29,30). In sporadic HB, this finding seems to be limited. Of note, cisplatin usually leads to apoptosis, which is an immunologically silent form of cell death. Understanding the molecular basis of these differences may help in the management of these patients.
The breakthrough results of T cells genetically engineered in patients with B-cell lymphomas have paved the way to adopt this therapy in solid tumors (31). Chimeric antigen receptors (CARs) consist of antigen binding sites linked to activating molecules. The antigen binding site is composed by the variable regions of a monoclonal antibody joined in a single chain. The intracellular component, whose function is to expand the engineered cells, include the T cell receptor-associated CD3 complex molecules: ζ chain, CD28, 4-1BB. CARs are transfected and expressed in T cells using retroviral vectors. In optimal conditions, when the transfected cells are cytotoxic effectors, the interaction with the tumor cells expressing the target antigens leads to tumor cell necrosis. CAR-T based therapy in patients with HB is at the outset.
At least two antigens suitable for CAR-T therapy have been identified on the surface of tumor cells: glypican-3 and EpCam. Glypican-3 is a co-receptor of WNT whose function is the control of cell division and growth. It is expressed in a large proportion of epithelial subtypes of HB and is absent in normal liver (32). Redirecting T cells to glypican-3 through CAR has led to necrosis of HB cell lines (33). EpCam is a transmembrane glycoprotein expressed in HB (13,34). Ep-Cam specific monoclonal antibodies elicit HB cell necrosis by γδ t cells (35). A phase 1 trial aimed at evaluating safety and efficacy of CAR-T cells redirected to EpCam in multiple tumor types is now recruiting patients.
Relevance of in vivo models
Generation of experimental models, especially in vivo, represents an important step both to better understand the histopathological and molecular events responsible for a certain disease as well as to design novel and effective therapeutic approaches. As concerns HB, a limited number of in vivo models have been established so far. This is presumably and mainly due to the paucity of funding available for this rare disease. In addition, the misclassification of some rodent liver tumors in HCCs rather than in HBs cannot be excluded. In the last two decades, the central role of the WNT/β-catenin pathway in human hepatocellular tumors has led to the development of transgenic mice overexpressing mutated forms of β-catenin in the liver. Unexpectedly, β-catenin activation alone was found to be insufficient to promote liver carcinogenesis in mouse models. Indeed, mice with liver-specific overexpression of either wild-type or oncogenic forms of the β-catenin gene failed to develop spontaneous liver cancer (36,37). However, mice with the activation of β-catenin signaling were found to be more susceptible to diethylnitrosamine-induced hepatocarcinogenesis (37), implying β-catenin mutation as a predisposing condition to HCC development in mice. On the other hand, HCC development was detected in mice with liver-targeted disruption of APC, which were characterized by strong activation of the β-catenin cascade (38). Unfortunately, a deep histopathological investigation was not conducted in these mice, which were classified as HCCs. Thus, it cannot be excluded that they are instead HBs or, at least, that they harbor some of the histopathological and/or molecular alterations of human HBs. Furthermore, it has been demonstrated that β-catenin cooperates with other oncogenes to induce HCC formation. For instance, mice harboring mutations of β-catenin and H-Ras in the liver developed HCC (39). Similarly, the concomitant overexpression of c-Met or an activated form of G12D-KRAS and mutated forms of β-catenin in the mouse liver via hydrodynamic gene delivery triggered HCC formation (40,41). In particular, these mice developed HCC with gene expression profiles that displayed high correlation with the gene profiles of a subset of human HCC patients with both CTNNB1 mutations and c-Met activation signatures (41). The importance of β-catenin in the oncogenic process was underscored by the finding that treatment of these mice with lipid nanoparticles containing small interfering RNA (siRNA) against CTNNB1 triggered a remarkable reduction of tumor burden (41). Based on the finding that the vast majority of human HB specimens exhibit concomitant nuclear accumulation of β-catenin and the YAP protooncogenes (22), a mouse model co-expressing constitutively active β-catenin and YAP forms via hydrodynamic gene delivery was generated. Of note, the simultaneous overexpression of β-catenin and YAP resulted in rapid HB development, whereas no tumors occurred when either mutant β-catenin or active Yap was overexpressed alone (22). Some molecular aspects of this mouse model were subsequently elucidated. Specifically, it has been shown that Yap activity in HB depends on its ability to bind the TEA domain transcription factors (TEADs), as either mutant forms of Yap that cannot bind TEADs or a dominant negative form of TEAD factors failed to induce tumor development when co-expressed with mutant β-catenin (22,42). In particular, TEAD4 was identified as the main member of this family of transcription factors in mediating Yap oncogenic potential in mouse HB (39). In a further investigation, it was found that the mammalian target of rapamycin complex 1 (mTORC1) signaling, involved in many functions of normal and neoplastic cells, is activated in human HB cells and YAP/β-catenin mice. At the molecular level, mTORC1 activation was induced by the amino acid transporter SLC38A1 in HB cell lines and in mouse HB tissues in a Yap-dependent manner (39). Intriguingly, it was more recently found that also β-catenin induces the activation of mTORC1 via Glutamine Synthase (43). Therefore, Yap and β-catenin converge on the activation of the mTORC1 pathway in HB (Figure 3). The importance of mTORC1 pathway in YAP/β-catenin-driven HB development was further substantiated in a study showing that a Rapamycin (an mTORC1 inhibitor) fed-diet mice significantly decreased HB burden when compared to controls (44,45). Altogether, these data suggest that targeting the mTORC1 signaling might represent a novel therapeutic strategy for the treatment of human HB.
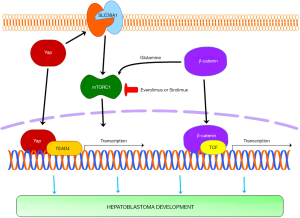
To the best of our knowledge, few additional mouse models of HB have been generated. Specifically, the c-Myc conditional mouse model is characterized by the development of neoplastic lesions resembling the main features of human HB (46). Of note, inactivation of c-Myc in these lesions led to tumor regression in these mice, implying the requirement of an active c-Myc for the tumors to grow (46). The importance of Myc proteins in HB has been further underscored by the finding that treatment with Bromodomain and Extra-Terminal motif (BET) and Aurora inhibitors targeting Myc are able to restrain the growth of HB cells in vitro (47). The second model consisted instead of the conditional transgenic mouse overexpressing the LIN28B RNA-binding protein, which is involved in stem cell maintenance, metabolism, and oncogenesis (48). In these mice, LIN28B overexpression was sufficient to initiate HB and HCC development. Of note, Lin28b overexpression was also detected in c-Myc-driven mouse HB, and liver-specific deletion of Lin28a/b significantly decreased tumor burden and delayed tumor onset, thus extending mouse survival (48). An additional model was generated by combining c-Myc and a dominant mutant allele of β-catenin (49). In this model, neonatal mice preferentially developed HB over other hepatocellular malignant lesions. Of note, the HB lesions were characterized by a strong induction of the nuclear factor (erythroid-derived 2)-like2/ NFE2L2/NRF2-dependent antioxidant signaling, which was specifically associated with the expression of β-catenin but not c-Myc. In the Hep2933 human HB cell line, silencing of NFE2L2/NRF2 gene was accompanied by a pronounced growth restraint, further substantiating the role of NFE2L2/NRF2 in HB tumorigenesis. Taken together, and considering that activating mutations of NFE2L2/NRF2 are the second most frequent alteration in human HB after those affecting CTNNB1 (18,19), these intriguing findings indicate that induction of the NRF2-driven antioxidant pathway by β-catenin overexpression might be one of the pivotal mechanisms allowing β-catenin activated cells to survive and expand in HB (49).
Altogether, although presumably incomplete, the available data on mouse models indicate the functional importance of YAP, β-catenin, mTORC1, c-Myc, LIN28a/b, and NFE2L2/NRF2 in HB development and suggest the usefulness of targeting these genes and/or the related pathways for the treatment of the human disease.
Conclusions
Different from many other solid tumor types, a striking improvement in HB treatment and relative patients’ survival has been achieved in the last decades by proper stratification of the patients and appropriate therapeutic strategies. However, some HB subsets are still associated with a poor prognosis and require more effective therapies. Some preclinical models have been recently generated showing the pathogenetic relevance of YAP, β-catenin, c-Myc, LIN28a/b, and NRF2 protooncogenes as well as the in vivo efficacy of mTORC1 inhibitors on the growth of HB experimental models. Further studies should be conducted to determine whether drugs targeting the aforementioned pathways are beneficial for the treatment of human HB.
Acknowledgments
Funding: This work was supported by Fondazione di Sardegna [Grant 2014-0188].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Aronson DC, Schnater JM, Staalman CR, et al. Predictive value of the pretreatment extent of disease system in HB: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol 2005;23:1245-52. [Crossref] [PubMed]
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric HB: a unified analysis from the Children's Hepatic Tumors International Collaboration. Lancet Oncol 2017;18:122-31. [Crossref] [PubMed]
- Sharma D, Subbarao G, Saxena R. HB. Semin Diagn Pathol 2017;34:192-200. [Crossref] [PubMed]
- Dong R, Zheng S, Dong K. Distinguishing Among Pediatric HBs, Transitional Liver Cell Tumors, and Hepatocellular Carcinomas and Using Appropriate Chemotherapy Regimens. J Clin Oncol 2017;35:115-6. [Crossref] [PubMed]
- Brown J, Perilongo G, Shafford E, et al. Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 2000;36:1418-25. [Crossref] [PubMed]
- Zsiros J, Brugieres L, Brock P, et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk HB (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol 2013;14:834-42. [Crossref] [PubMed]
- Katzenstein HM, Langham MR, Malogolowkin MH, et al. Minimal adjuvant chemotherapy for children with hepatoblastoma resected at diagnosis (AHEP0731): a Children's Oncology Group, multicentre, phase 3 trial. Lancet Oncol 2019;20:719-27. [Crossref] [PubMed]
- Paediatric Hepatic International Tumor Trial. Available online: https://www.birmingham.ac.uk/Documents/college-mds/trials/crctu/phitt/Protocol/Current/PHITT-Protocol-version-3-0-17Oct2018.pdf
- Marin JJG, Cives-Losada C, Asensio M, et al. Mechanisms of Anticancer Drug Resistance in Hepatoblastoma. Cancers (Basel) 2019;11:1-20. [Crossref] [PubMed]
- Koch A, Denkhaus D, Albrecht S, et al. Childhood HBs frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res 1999;59:269-73. [PubMed]
- Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J 2012;31:2670-84. [Crossref] [PubMed]
- Monga SP. β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology 2015;148:1294-310. [Crossref] [PubMed]
- Cairo S, Armengol C, De Reyniès A, et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell 2008;14:471-84. [Crossref] [PubMed]
- Sumazin P, Chen Y, Treviño LR, et al. Genomic analysis of HB identifies distinct molecular and prognostic subgroups. Hepatology 2017;65:104-21. [Crossref] [PubMed]
- Hooks KB, Audoux J, Fazli H, et al. New insights into diagnosis and therapeutic options for proliferative HB. Hepatology 2018;68:89-102. [Crossref] [PubMed]
- Vokuhl C, Oyen F, Häberle B, et al. Small cell undifferentiated (SCUD) HBs: All malignant rhabdoid tumors? Genes Chromosomes Cancer 2016;55:925-31. [Crossref] [PubMed]
- Scotting PJ, Walker DA, Perilongo G. Childhood solid tumours: a developmental disorder. Nat Rev Cancer 2005;5:481-8. [Crossref] [PubMed]
- Eichenmüller M, Gruner I, Hagl B, et al. Blocking the hedgehog pathway inhibits HB growth. Hepatology 2009;49:482-90. [Crossref] [PubMed]
- Jia D, Dong R, Jing Y, et al. Exome sequencing of HB reveals novel mutations and cancer genes in the Wnt pathway and ubiquitin ligase complex. Hepatology 2014;60:1686-96. [Crossref] [PubMed]
- López-Terrada D, Gunaratne PH, Adesina AM, et al. Histologic subtypes of HB are characterized by differential canonical Wnt and Notch pathway activation in DLK+ precursors. Hum Pathol 2009;40:783-94. [Crossref] [PubMed]
- Marini KD, Payne BJ, Watkins DN, et al. Mechanisms of Hedgehog signalling in cancer. Growth Factors 2011;29:221-34. [Crossref] [PubMed]
- Tao J, Calvisi DF, Ranganathan S, et al. Activation of β-catenin and Yap1 in human HB and induction of hepatocarcinogenesis in mice. Gastroenterology 2014;147:690-701. [Crossref] [PubMed]
- Katoh M, Katoh M. Molecular genetics and targeted therapy of WNT-related human diseases Int J Mol Med 2017;40:587-606. (Review). [PubMed]
- Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev 2018;62:50-60. [Crossref] [PubMed]
- Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015;12:445-64. [Crossref] [PubMed]
- Liu P, Calvisi DF, Kiss A, et al. Central role of mTORC1 downstream of YAP/TAZ in HB development. Oncotarget 2017;8:73433-47. [PubMed]
- Clinical Trials.gov. Available online: https://clinicaltrials.gov
- Morcrette G, Hirsch TZ, Badour E, et al. APC germline HBs demonstrate cisplatin-induced intratumor tertiary lymphoid structures. Oncoimmunology 2019;8:e1583547. [Crossref] [PubMed]
- Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005;202:1691-701. [Crossref] [PubMed]
- Galluzzi L, Buqué A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017;17:97-111. [Crossref] [PubMed]
- Dotti G, Savoldo B, Brenner M. Fifteen years of gene therapy based on chimeric antigen receptors: "are we nearly there yet? Hum Gene Ther 2009;20:1229-39. [Crossref] [PubMed]
- Xiong XL, Qin H, Yan SQ, et al. Expression of glypican-3 is highly associated with pediatric hepatoblastoma: a systemic analysis. Asian Pac J Cancer Prev 2015;16:1029-31. [Crossref] [PubMed]
- Shimizu Y, Suzuki T, Yoshikawa T, et al. Next-Generation Cancer Immunotherapy Targeting Glypican-3. Front Oncol 2019;9:248. [Crossref] [PubMed]
- Kiruthiga KG, Ramakrishna B, Saha S, et al. Histological and immunohistochemical study of hepatoblastoma: correlation with tumour behavior and survival. J Gastrointest Oncol 2018;9:326-37. [Crossref] [PubMed]
- Armeanu-Ebinger S, Hoh A, Wenz J, et al. Targeting EpCAM (CD326) for immunotherapy in hepatoblastoma. Oncoimmunology 2013;2:e22620. [Crossref] [PubMed]
- Harada N, Miyoshi H, Murai N, et al. Lack of tumorigenesis in the mouse liver after adenovirus-mediated expression of a dominant stable mutant of beta-catenin. Cancer Res 2002;62:1971-7. [PubMed]
- Nejak-Bowen KN, Thompson MD, Singh S. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology 2010;51:1603-13. [Crossref] [PubMed]
- Colnot S, Decaens T, Niwa-Kawakita M, et al. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci U S A 2004;101:17216-21. [Crossref] [PubMed]
- Harada N, Oshima H, Katoh M, et al. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res 2004;64:48-54. [Crossref] [PubMed]
- Tao J, Xu E, Zhao Y, et al. Modeling a human hepatocellular carcinoma subset in mice through coexpression of met and point-mutant β-catenin. Hepatology 2016;64:1587-605. [Crossref] [PubMed]
- Tao J, Zhang R, Singh S, et al. Targeting β-catenin in hepatocellular cancers induced by coexpression of mutant β-catenin and K-Ras in mice. Hepatology 2017;65:1581-99. [Crossref] [PubMed]
- Zhang J, Liu P, Tao J, et al. TEA Domain Transcription Factor 4 Is the Major Mediator of Yes-Associated Protein Oncogenic Activity in Mouse and Human Hepatoblastoma. Am J Pathol 2019;189:1077-90. [Crossref] [PubMed]
- Adebayo Michael AO, Ko S, Tao J, et al. Inhibiting Glutamine-Dependent mTORC1 Activation Ameliorates Liver Cancers Driven by β-Catenin Mutations. Cell Metab 2019;29:1135-50.e6. [Crossref] [PubMed]
- Molina L, Yang H, Adebayo Michael AO, et al. mTOR inhibition affects Yap1-β-catenin-induced hepatoblastoma growth and development. Oncotarget 2019;10:1475-90. [PubMed]
- Russell JO, Monga SP. Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu Rev Pathol 2018;13:351-78. [Crossref] [PubMed]
- Shachaf CM, Kopelman AM, Arvanitis C, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer Nature 2004;431:1112-7. [Crossref] [PubMed]
- Eberherr C, Beck A, Vokuhl C, et al. Targeting excessive MYCN expression using MLN8237 and JQ1 impairs the growth of hepatoblastoma cells. Int J Oncol 2019;54:1853-63. [PubMed]
- Nguyen LH, Robinton DA, Seligson MT, et al. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell 2014;26:248-61. [Crossref] [PubMed]
- Comerford SA, Hinnant EA, Chen Y, et al. Hepatoblastoma modeling in mice places Nrf2 within a cancer field established by mutant β-catenin. JCI Insight 2016;1:e88549. [Crossref] [PubMed]
Cite this article as: Calvisi DF, Solinas A. Hepatoblastoma: current knowledge and promises from preclinical studies. Transl Gastroenterol Hepatol 2020;5:42.