EUS guided gallbladder drainage
Introduction
Acute cholecystitis is acute inflammation of the gallbladder, most commonly treated with open or laparoscopic cholecystectomy. In most cases, inflammation arises from gallstones, but only 1% to 2% of individuals with gallstones become symptomatic annually (1). Acute cholecystitis, the leading complication, can develop in up to 10% percent of these patients (1). Although surgery is the gold standard for treatment, some patients are poor surgical candidates due to high-risk comorbidities including cirrhosis, ascites, coagulopathy, cancers, and cardiopulmonary conditions.
Percutaneous gallbladder drainage (PT-GBD) has been described since the 1970’s, and is typically performed as an alternative in these cases. Technical success rates are good, ranging from 95% to 100% (2). Although PT-GBD is the most widely established alternative to cholecystectomy, it has a complication rate of up to 12%, including puncture-induced hemorrhage, pneumothorax, bile peritonitis, and drain site pain or infection (3-5). The procedure is not recommended in patients with perihepatic ascites, intervening loops of bowel, coagulopathy, and when there is concern for nonadherence (6). In approximately 40% of patients, it is primarily only a temporizing measure, and definitive therapy may not ultimately be pursued (7). In patients who cannot undergo surgery, necessary removal of the catheter often results in recurrent cholecystitis (8).
As an alternative, endoscopic techniques for short- or long-term therapy for cholecystitis have been described. Endoscopic transpapillary gallbladder drainage (ET-GBD) has comparable efficacy with percutaneous drainage and shorter hospitalization periods (9). However, this procedure can be technically challenging or fail since cystic duct obstruction by either inflammation or a stone is common. In a series of 43 patients who underwent ET-GBD, a technical success rate of only 84% was achieved, mainly due to difficulty maneuvering the guidewire into the gallbladder and when advancing the drainage catheter into the gallbladder (10).
In an attempt to overcome these challenges, Baron and colleagues described endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) in 2007, which has since shown novel success in patients with risk factors for cholecystectomy (11). EUS-GBD can be used for both gallbladder drainage and gallstone removal, although recurrence can still occur as long as the gallbladder remains in situ (12). ET-GBD and EUS-GBD are therefore becoming promising alternatives. When comparing ET-GBD and EUS-GBD, Khan and colleagues demonstrated that technical and clinical success rates of EUS-GBD were superior to ET-GBD (13).
Clinical indications for EUS-GBD
While EUS-GBD was initially introduced as an alternative to surgery for patients who are considered non-surgical candidates, its applications continue to evolve. The current indications for EUS-guided gallbladder drainage in patients with acute cholecystitis include (I) nonsurgical candidates with and without stone extraction, (II) bridging therapy to cholecystectomy, (III) conversion from PT-GBD to EUS-GBD, (IV) alternative to failed PT-GBD or ET-GBD, or (V) alternative to failed EUS-guided biliary drainage (EUS-BD) (12).
Patients who are not currently optimized for surgery, but in whom surgery could be considered in the future may consider EUS-GBD as a bridge to cholecystectomy (12,14). As the number of EUS-GBD cases increases in these patients, consensus guidelines should be established between endoscopists and surgeons. For surgeons, closing the anastomotic site from the gastrointestinal wall can be challenging, and transgastric drainage may be preferred.
Patients may also opt to pursue EUS-GBD for conversion from prior PT-GBD since internal drainage may be less painful and more cosmetically pleasant than percutaneous drainage (8,12,15,16). Recently, a multicenter case series of the conversion of PT-GBD to internal transmural EUS-GBD was described by Minaga et al. The technical success rate was reported as 90%. The percutaneous drains were removed in 17/21 patients. Reintervention was required in two patients due to stent occlusion and migration (17). The technical and clinical success rates are promising, but this is a small study, and additional studies are needed in this area. EUS-GBD can also be used as an alternative to failed PT-GBD or ET-GBD when anatomic and technical issues are present (12).
Lastly, EUS-GBD can be used as an alternative to failed EUS guided biliary access. EUS-BD is used in expert centers when conventional ERCP fails. If EUS-BD is unsuccessful, and the gallbladder has a connection with the proximal bile duct via the cystic duct, gallbladder drainage may provide some biliary decompression as a salvage technique (12,18).
Recent reviews of all EUS-GBD cases have demonstrated technical and clinical success rates greater than 95% and 93%, respectively (1,19,20). Khan and colleagues demonstrated that this procedure has superior clinical and technical success rates as compared to PT-GBD while requiring less interventions (13). Patients who underwent PT-GBD had greater clinical success and showed shorter hospitalization periods, fewer repeat interventions, and less adverse advents (21,22). Therefore, EUS-GBD is a minimally-invasive alternative that can be used as definitive therapy in patients with acute cholecystitis.
Methods
Procedure considerations
EUS-GBD is being employed at increasing numbers of tertiary centers worldwide by experts in both EUS and endoscopic retrograde cholangiopancreatography (ERCP). If patients are considered to be poor surgical candidates due to comorbidities, it is appropriate to consider evaluation for endoscopic drainage. Patients who may undergo cholecystectomy in the near future can be considered for either PT-GBD or endoscopic drainage as a temporizing measure. Therefore, it is appropriate to have a multidisciplinary discussion with surgery, interventional radiology, and gastroenterology, since there are varying levels of expertise regionally.
Patient selection and evaluation
Nonsurgical candidates needing definitive therapy for cholecystitis should be considered for EUS-GBD. There should be comprehensive imaging analysis prior to the procedure. The patient should give specific informed consent for EUS-GBD after a thorough discussion of the risks, benefits, and alternatives to the procedure.
Materials and instruments
All endoscopic procedures are performed with monitored anesthesia care or general anesthesia. Patients should be given antibiotics prior to the procedure. A curved linear-array echoendoscope is used to visualize the gallbladder and color flow Doppler is used to identify regional vasculature prior to puncture.
Selection of access site (choice of approach)
The gallbladder can be accessed from the gastrointestinal tract by both the distal gastric antrum and duodenal bulb. This is typically left to the discretion of the endoscopist, based upon evaluation of the patient’s anatomy in order to determine the site of maximal direct apposition between the gallbladder and GI tract wall. The retroperitoneal location of the duodenum is less mobile, providing a safer puncture site at the gallbadder neck, thus making it easier and preferable for endoscopists (23). Walter and colleagues showed that it might also allow for more stable tract formation as compared to the stomach where peristalsis can lead to a higher degree of tissue overgrowth (24). Transduodenal access may also carry a lower risk of stent migration or dislodgement in the long term and may be associated with lower risk of food reflux into the gallbladder (23).
Transgastric access aims for the gallbladder body, which is a larger entry point and easier target, particularly during stent deployment. Transgastric access may also be favored in patients with tumor infiltration of the duodenum or with duodenal self-expanding metal stents, which is common since many of these patients have pancreaticobiliary malignancy (23). In terms of adverse events, the consequences may be less serious since surgical access to the stomach is easier than the duodenal bulb. For this reason, in patients who will ultimately undergo cholecystectomy, surgeons typically favor the transgastric approach since fistula closure is easier, although a fibrous band between the stomach and gallbladder may make cholecystectomy more challenging (23).
Detailed evaluations of each access site should be performed and patients’ individual anatomy and long-term needs should be considered (23,25,26). The approach with better endosonographic imaging of the gallbladder, closer apposition, lack of interposing vessels, and stable scope position should be favored since to date, there are no differences in technical or clinical success rates and in the incidence of adverse events between these two approaches (27). Early data on cholecystectomy after EUS-GBD is promising, but further studies addressing these details are needed. In a study by Saumoy and colleagues, 13 patients who previously underwent EUS-GBD underwent technically successful cholecystectomy (14).
Technique
Once the site of puncture has been determined, the distance from the luminal wall and the gallbladder is measured (Figure 1). A 19-guage needle is used to puncture the gallbladder, and contrast is injected to confirm location (Figure 2). A 0.025-inch or 0.035-inch guidewire is then passed through the needle and coiled into the gallbladder. The fistula can be dilated using a bougie (6F or 7F) or tapered tip balloon dilator (4 mm) (15,25). In instances where there is resistance to advancing the 6F bougie, a needle-knife or a cystostomy can be used (25). When more than one stent will be placed, a second guidewire can be used with a 4 or 6 mm balloon dilator to further dilate the tract (15).
An alternative to the above-mentioned steps is to proceed directly with placement of a LAMS (lumen apposing metal stent) using a single-step electrocautery-enhanced delivery system (Hot AXIOSTM, Boston Scientific, Marlboro, MA USA), which will be discussed in more detail.
Stent selection
EUS-guided drainage techniques have previously been limited by available accessories. Plastic double pigtail stents have long-served drainage purposes, but bile leaks are common due to their small diameter, and they are associated with potential complications such as pneumoperitoneum, bile peritonitis, and stent migration. Nasocystic drainage catheters have been preferred by some endoscopists, however their maintenance is challenging for both patients and nursing staff (28).
The emergence of self-expandable metal stents (SEMS) alleviated this problem, allowing for prolonged stent patency (29). Khan et al. showed that using a plastic stent or naso-gallbladder drainage catheter is more likely to have adverse events as compared with EUS-GBD using a metal stent, so there is now a preference for metal stents (13). Due to expandability, a metal stent can seal the gap between the stent and the fistula of the gallbladder wall, preventing bile leakage (30).
While the use of fully covered biliary metal stents may reduce the risk of bile leak, the stents don’t maintain apposition between the gallbladder and the GI tract in order to form a secure fistula. They may also be too long for optimal positioning and can impinge on adjacent structures (23). Stent migration is also a concern for fully covered biliary metal stents, due to their large diameter.
Modifications to conventional metal stents have reduced these previously associated risks. Fully covered SEMS with anti-migratory fins were described to prevent tissue ingrowth and stent displacement (31). Itoi et al. first described the AXIOS fully covered metal stent, which allows for lumen apposition due to its bilateral anchor flanges (32). However, there are still some concerns regarding the use of metal stents, particularly in patients who may later undergo elective cholecystectomy.
Lumen-apposing metal stents (LAMS) are self-expanding, saddle-shaped, silicone covered stents, which are ideal for EUS-GBD. These stents have an ability to provide anchorage in non-adherent luminal structures, prevent tissue ingrowth and tract leakage, and can be removed (1). The stent has bilateral anchor flanges and is delivered through a 10.5F catheter, allowing for a two-step release of each flange and prevention of unintended deployment (32). There are various types of lumen apposing metal stents. de La Serna and colleagues reported a study comparing three lumen apposing stents, AXIOS (Boston Scientific, Mattick, MA, USA), NAGI (Taewoong, Gimpo, Korea) and SPAXUS (Taewoong, Gimpo, Korea). The lumen apposing force for both the AXIOS and SPAXUS were superior to the NAGI (33).
The Hot AXIOS electrocautery enhanced stent delivery system mentioned previously was introduced in an attempt to streamline the drainage process into one accessory, as the repeated instrument exchanges previously required increases the risk of adverse events (24,34). Gallbladder access can be achieved either with a 19-gauge needle or directly with the electrocautery enhanced device as deemed appropriate by the endoscopist. When a 19-gauge needle is used, a guidewire is advanced through the needle and coiled within the gallbladder and the needle is exchanged for the stent deployment system. Otherwise, the deployment system can be used in a freehand technique without a guidewire. The system is advanced across the fistula tract using electrocautery. The distal flange is deployed under endosonographic guidance, while the proximal flange is deployed under EUS or endoscopic guidance. In the largest series with LAMS to date, the mean stent deployment time using this system was 3.1 minutes as compared with the mean time of 7.7 minutes necessary with the over the wire stent insertion (35). It should be noted that most operators experienced in this one-step, freehand technique have had extensive experience with the over the wire technique, which lead to their evolutionary experience with the free hand technique. There is some concern with novel operators and the reproducibility of this method.
Follow up
Several options can be pursued after EUS-GBD. In patients with minimal life expectancy, the AXIOS stent can be left indefinitely and can act as definitive therapy for a few months (23). Metal stents provide good long-term results, with only 7% experiencing stent migration and/or relapsing cholecystitis (23). These results are similar in both tubular covered SEMS and in LAMS (24,35,36). In patients requiring long-term intervention, surgery remains the gold standard should they become surgical candidates. Preliminary data suggests that LAMs cause minimal or no interference with subsequent cholecystectomy, but until more evidence is available, plastic stents or nasocystic catheters can be considered. If surgery remains high-risk, the metal stent may be left in indefinitely or there can be a stent revision one month after EUS-GBD. The LAMS can be replaced with a double-pigtail plastic stent, which avoids stent migration and food impaction into the gallbladder (37). The double-pigtail stent may be left indefinitely.
Case series
Technical success and outcomes
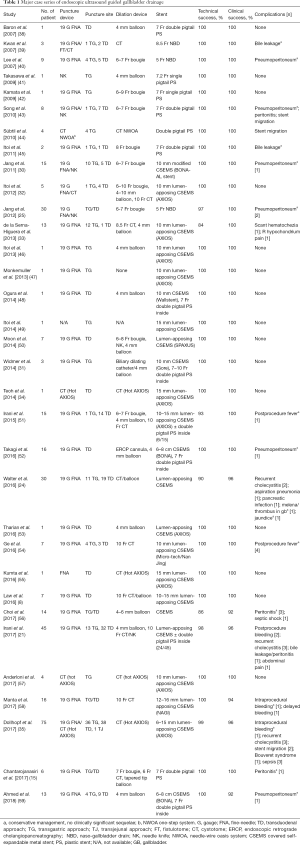
When pooling the data from large case series of EUS-GBD described in Table 1, the technical success of EUS-GBD is described in the literature as 84% to 100%, with successful stent deployment seen in 346 patients out of a series of 357 patients. Among technically successful procedures, 337 patients showed clinical success. This is a promising overall clinical success rate of approximately 97% for EUS-GBD when compared to PT-GBD, which has clinical success rates ranging from 56% to 100% (60).
Full table
Adverse events
Common adverse events with EUS-GBD include pneumoperitoneum, stent migration, bile leak, bile peritonitis, and bleeding. The most common is pneumoperitoneum, which has a speculated association with the sheer force of tract dilation on the gallbladder wall (61). Minimal dilation is therefore suggested to avoid this risk (20,61). Plastic stents may increase the risk of bile leak due to their small diameter; self-expanding metal stents should be used, as they allow for automatic sealing of the gap between the stent and the walls of the fistula (30,35). The use of self-expanding LAMS allows for minimal dilation and decreases the risk of stent migration. By reducing the risk of stent migration, the risk of bile leakage also decreases (43,60,62). Intraprocedural bleeding can occur during EUS-GBD when the transmural fistula is created between the gastrointestinal tract and gallbladder wall (1). In one series, Lang et al. found that although LAMS and double pigtail plastic stents had similar rates of clinical success, there was a significantly greater number of adverse events when using LAMS, specifically bleeding and unplanned endoscopies (63). The risk of stent misdeployment remains a challenge for endoscopists during EUS-GBD. For novel endoscopists, the introduction of the Hot AXIOS stent and its “free-hand” technique may increase the risk of stent misdeployment (23). Using a guidewire may appropriate for new operators in order to decrease the risk of cautery-induced injury to the contralateral gallbladder wall and provide a salvage method in the event of stent misdeployment (23). However, in some reported cases, EUS-GBD is not technically successful due to loss of guidewire access (25,33,56). The Hot AXIOS deployment system may be therefore be beneficial, since it eliminates the need for initial needle puncture and guidewire placement, in addition to the need for tract dilation (64).
Role in clinical practice
The endoscopist performing EUS-GBD should have expertise in both EUS and ERCP. There is a lack of opportunity for trainees to obtain extensive exposure to these procedures during fellowship, but this foundation should be required for those wishing to pursue therapeutic EUS later in practice (65). The first step for training is to become familiarized with conventional EUS-guided FNA, celiac plexus block, and pseudocyst drainage (65). After ideally more than 20 cases of EUS-guided pseudocyst drainage, the trainee may then practice on more difficult targets like the gallbladder with the assistance of an experienced mentor (65). EUS-GBD is technically challenging because the gallbladder is a mobile structure and, unlike pancreatic pseudocysts, is not adherent to the gastric wall. The training required to perform the procedure is therefore more comprehensive than that required for EUS-guided drainage of pancreatic fluid collections, but one way for trainees to develop the required skills is to perform on easier targets like pseudocysts (65).
Future considerations
In patients who cannot undergo cholecystectomy, EUS-GBD has been successfully described as both definitive therapy and a bridge to surgery. For those with minimal life expectancies or high-risk comorbidities, the initial placement of a LAMS (e.g., AXIOS) by EUS-GBD has allowed for definitive therapy (24,66). The need for an additional double-pigtail plastic stent inside remains unclear (66). Should stents be removed after resolution of acute cholecystitis? What is the optimal duration of stenting? Studies show minimal adverse events even up to three years with SEMS and LAMS suggesting long-term stenting is a viable option (3,30,36,66). Alternatively, for patients who require long-term treatment, the LAMS can be replaced after approximately one month by a double-pigtail plastic stent, which can be left indefinitely. This exchange has been successful in avoiding possible stent migration and food impaction (37).
The evolution of accessories specifically designed for EUS-GBD will further reduce the risk of adverse events associated with the procedure. Technical and clinical success rates should also see improvement. Authors believe that accessories like the Hot AXIOS deployment system will be beneficial because it decreases the number of accessories exchanged, potentially reducing the frequency of complications (24).
If the patient becomes an appropriate candidate for cholecystectomy at any time, the option should be explored, since it eliminates the risk of recurrent acute cholecystitis. There is limited discussion of cholecystectomy after EUS-GBD, but the process has been successful in reported cases (25,66). As EUS-GBD becomes more widely adopted, there should be consideration for developing techniques that optimize subsequent surgery outcomes.
EUS-GBD is overall a promising technique, which is being employed at increasing numbers at expert centers internationally. With impressive technical and clinical success rates with low rates of adverse events, it should be considered for non-surgical candidates with acute cholecystitis. Its applications continue to expand, along with the evolution of accessories to streamline the procedure. It should be considered as a mainstay of therapy for appropriate candidates.
Acknowledgments
None.
Footnote
Conflicts of Interest: Jessica Widmer: Boston Scientific, consultant. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Jain D, Bhandari BS, Agrawal N, et al. Endoscopic Ultrasound-Guided Gallbladder Drainage Using a Lumen-Apposing Metal Stent for Acute Cholecystitis: A Systematic Review. Clin Endosc 2018;51:450-62. [Crossref] [PubMed]
- Akhan O, Akıncı D, Özmen MN. Percutaneous cholecystostomy. Eur J Radiol 2002;43:229-36. [Crossref] [PubMed]
- Itoi T, Coelho-Prabhu N, Baron TH. Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest Endosc 2010;71:1038-45. [Crossref] [PubMed]
- McGahan JP, Lindfors KK. Percutaneous cholecystostomy: an alternative to surgical cholecystostomy for acute cholecystitis? Radiology 1989;173:481-5. [Crossref] [PubMed]
- Winbladh A, Gullstrand P, Svanvik J, et al. Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB (Oxford) 2009;11:183-93. [Crossref] [PubMed]
- Small AJ, Irani S. EUS-guided gallbladder drainage vs. percutaneous gallbladder drainage. Endosc Ultrasound 2018;7:89-92. [PubMed]
- Al-Jundi W, Cannon T, Antakia R, et al. Percutaneous cholecystostomy as an alternative to cholecystectomy in high risk patients with biliary sepsis: a district general hospital experience. Ann R Coll Surg Engl 2012;94:99-101. [Crossref] [PubMed]
- Law R, Grimm IS, Stavas JM, et al. Conversion of Percutaneous Cholecystostomy to Internal Transmural Gallbladder Drainage Using an Endoscopic Ultrasound-Guided, Lumen-Apposing Metal Stent. Clin Gastroenterol Hepatol 2016;14:476-80. [Crossref] [PubMed]
- Iino C, Shimoyama T, Igarashi T, et al. Comparable efficacy of endoscopic transpapillary gallbladder drainage and percutaneous transhepatic gallbladder drainage in acute cholecystitis. Endosc Int Open 2018;6:E594-601. [Crossref] [PubMed]
- Itoi T, Sofuni A, Itokawa F, et al. Endoscopic transpapillary gallbladder drainage in patients with acute cholecystitis in whom percutaneous transhepatic approach is contraindicated or anatomically impossible (with video). Gastrointest Endosc 2008;68:455-60. [Crossref] [PubMed]
- Gan SI, Rajan E, Adler DG, et al. Role of EUS. Gastrointest Endosc 2007;66:425-34. [Crossref] [PubMed]
- Itoi T, Tsuchiya T, Sofuni A, et al. Development of EUS-guided gallbladder drainage and current indications. Endosc Ultrasound 2018;7:76-8. [PubMed]
- Khan MA, Atiq O, Kubiliun N, et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc 2017;85:76-87.e3. [Crossref] [PubMed]
- Saumoy M, Tyberg A, Brown E, et al. Successful Cholecystectomy After Endoscopic Ultrasound Gallbladder Drainage Compared With Percutaneous Cholecystostomy, Can it Be Done? J Clin Gastroenterol 2019;53:231-5. [Crossref] [PubMed]
- Chantarojanasiri T, Matsubara S, Isayama H, et al. Feasibility of conversion of percutaneous cholecystostomy to internal transmural endoscopic ultrasound-guided gallbladder drainage. Saudi J Gastroenterol 2017;23:318-22. [Crossref] [PubMed]
- Teoh AYB. Feasibility of the conversion of percutaneous cholecystostomy to internal trans-mural endoscopic ultrasound-guided gallbladder drainage. Saudi J Gastroenterol 2017;23:309-10. [Crossref] [PubMed]
- Minaga K, Yamashita Y, Ogura T, et al. Clinical Efficacy and Safety of EUS-guided Gallbladder Drainage Replacement of Percutaneous Drainage: A Multicenter Retrospective Study. Dig Endosc 2019;31:180-7. [Crossref] [PubMed]
- Imai H, Kitano M, Omoto S, et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc 2016;84:147-51. [Crossref] [PubMed]
- Manta R, Mutignani M, Galloro G, et al. Endoscopic ultrasound-guided gallbladder drainage for acute cholecystitis with a lumen-apposing metal stent: a systematic review of case series. Eur J Gastroenterol Hepatol 2018;30:695-8. [Crossref] [PubMed]
- Kalva NR, Vanar V, Forcione D, et al. Efficacy and Safety of Lumen Apposing Self-Expandable Metal Stents for EUS Guided Cholecystostomy: A Meta-Analysis and Systematic Review. Can J Gastroenterol Hepatol 2018;2018:7070961. [Crossref] [PubMed]
- Irani S, Ngamruengphong S, Teoh A, et al. Similar Efficacies of Endoscopic Ultrasound Gallbladder Drainage With a Lumen-Apposing Metal Stent Versus Percutaneous Transhepatic Gallbladder Drainage for Acute Cholecystitis. Clin Gastroenterol Hepatol 2017;15:738-45. [Crossref] [PubMed]
- Tyberg A, Saumoy M, Sequeiros EV, et al. EUS-guided Versus Percutaneous Gallbladder Drainage: Isn't It Time to Convert? J Clin Gastroenterol 2018;52:79-84. [Crossref] [PubMed]
- Perez-Miranda M. Technical considerations in EUS-guided gallbladder drainage. Endosc Ultrasound 2018;7:79-82. [PubMed]
- Walter D, Teoh AY, Itoi T, et al. EUS-guided gall bladder drainage with a lumen-apposing metal stent: a prospective long-term evaluation. Gut 2016;65:6-8. [Crossref] [PubMed]
- Jang JW, Lee SS, Song TJ, et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology 2012;142:805-11. [Crossref] [PubMed]
- Jamwal KD, Sharma MK, Maiwall R, et al. EUS-guided Gall Bladder Drainage in Severe Liver Disease: A Single-center Experience in Critically Ill Cirrhotics. J Clin Transl Hepatol 2018;6:35-9. [PubMed]
- Teoh AYB, Serna C, Penas I, et al. Endoscopic ultrasound-guided gallbladder drainage reduces adverse events compared with percutaneous cholecystostomy in patients who are unfit for cholecystectomy. Endoscopy 2017;49:130-8. [PubMed]
- Saumoy M, Kahaleh M. Superiority of metal stents for pancreatic walled-off necrosis: bigger is better! Gastrointest Endosc 2017;85:1253-4. [Crossref] [PubMed]
- Ballard DD, Rahman S, Ginnebaugh B, et al. Safety and efficacy of self-expanding metal stents for biliary drainage in patients receiving neoadjuvant therapy for pancreatic cancer. Endosc Int Open 2018;6:E714-21. [Crossref] [PubMed]
- Jang JW, Lee SS, Park DH, et al. Feasibility and safety of EUS-guided transgastric/transduodenal gallbladder drainage with single-step placement of a modified covered self-expandable metal stent in patients unsuitable for cholecystectomy. Gastrointest Endosc 2011;74:176-81. [Crossref] [PubMed]
- Widmer J, Alvarez P, Gaidhane M, et al. Endoscopic ultrasonography-guided cholecystogastrostomy in patients with unresectable pancreatic cancer using anti-migratory metal stents: a new approach. Dig Endosc 2014;26:599-602. [Crossref] [PubMed]
- Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc 2012;75:870-6. [Crossref] [PubMed]
- de la Serna-Higuera C, Perez-Miranda M, Gil-Simon P, et al. EUS-guided transenteric gallbladder drainage with a new fistula-forming, lumen-apposing metal stent. Gastrointest Endosc 2013;77:303-8. [Crossref] [PubMed]
- Teoh AY, Binmoeller KF, Lau JY. Single-step EUS-guided puncture and delivery of a lumen-apposing stent for gallbladder drainage using a novel cautery-tipped stent delivery system. Gastrointest Endosc 2014;80:1171. [Crossref] [PubMed]
- Dollhopf M, Larghi A, Will U, et al. EUS-guided gallbladder drainage in patients with acute cholecystitis and high surgical risk using an electrocautery-enhanced lumen-apposing metal stent device. Gastrointest Endosc 2017;86:636-43. [Crossref] [PubMed]
- Choi JH, Lee SS, Choi JH, et al. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy 2014;46:656-61. [Crossref] [PubMed]
- Kamata K, Takenaka M, Kitano M, et al. Endoscopic ultrasound-guided gallbladder drainage for acute cholecystitis: Long-term outcomes after removal of a self-expandable metal stent. World J Gastroenterol 2017;23:661-7. [Crossref] [PubMed]
- Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc 2007;65:735-7. [Crossref] [PubMed]
- Kwan V, Eisendrath P, Antaki F, et al. EUS-guided cholecystenterostomy: a new technique (with videos). Gastrointest Endosc 2007;66:582-6. [Crossref] [PubMed]
- Lee SS, Park DH, Hwang CY, et al. EUS-guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high-risk patients: a prospective feasibility study. Gastrointest Endosc 2007;66:1008-12. [Crossref] [PubMed]
- Takasawa O, Fujita N, Noda Y, et al. Endosonography-guided gallbladder drainage for acute cholecystitis following covered metal stent deployment. Dig Endosc 2009;21:43-7. [Crossref] [PubMed]
- Kamata K, Kitano M, Komaki T, et al. Transgastric endoscopic ultrasound (EUS)-guided gallbladder drainage for acute cholecystitis. Endoscopy 2009;41 Suppl 2:E315-6. [Crossref] [PubMed]
- Song TJ, Park DH, Eum JB, et al. EUS-guided cholecystoenterostomy with single-step placement of a 7F double-pigtail plastic stent in patients who are unsuitable for cholecystectomy: a pilot study (with video). Gastrointest Endosc 2010;71:634-40. [Crossref] [PubMed]
- Súbtil JC, Betes M, Munoz-Navas M. Gallbladder drainage guided by endoscopic ultrasound. World J Gastrointest Endosc 2010;2:203-9. [Crossref] [PubMed]
- Itoi T, Itokawa F, Kurihara T. Endoscopic ultrasonography-guided gallbladder drainage: actual technical presentations and review of the literature (with videos). J Hepatobiliary Pancreat Sci 2011;18:282-6. [Crossref] [PubMed]
- Itoi T, Binmoeller K, Itokawa F, et al. Endoscopic ultrasonography-guided cholecystogastrostomy using a lumen-apposing metal stent as an alternative to extrahepatic bile duct drainage in pancreatic cancer with duodenal invasion. Dig Endosc 2013;25 Suppl 2:137-141. [Crossref] [PubMed]
- Monkemuller K, Zabielski M, Didowacz-Grollmann A, et al. Endoluminal transgastric endoscopic anastomosis of the gallbladder using an anchoring self-expanding metal stent. Endoscopy 2013;45 Suppl 2 UCTN:E164-6.
- Ogura T, Masuda D, Imoto A, et al. EUS-guided gallbladder drainage and hepaticogastrostomy for acute cholecystitis and obstructive jaundice (with video). Endoscopy 2014;46 Suppl 1 UCTN:E75-6.
- Itoi T, Itokawa F, Tsuchiya T, et al. Transgastric large gallstone extraction through a lumen-apposing metal stent in a patient with acute cholecystitis. Gastrointest Endosc 2014;79:547. [Crossref] [PubMed]
- Moon JH, Choi HJ, Kim DC, et al. A newly designed fully covered metal stent for lumen apposition in EUS-guided drainage and access: a feasibility study (with videos). Gastrointest Endosc 2014;79:990-5. [Crossref] [PubMed]
- Irani S, Baron TH, Grimm IS, et al. EUS-guided gallbladder drainage with a lumen-apposing metal stent (with video). Gastrointest Endosc 2015;82:1110-5. [Crossref] [PubMed]
- Takagi W, Ogura T, Sano T, et al. EUS-guided cholecystoduodenostomy for acute cholecystitis with an anti-stent migration and anti-food impaction system; a pilot study. Therap Adv Gastroenterol 2016;9:19-25. [Crossref] [PubMed]
- Tharian B, Varadarajulu S, Hawes R. Drainage of obstructed gallbladder with use of lumen-apposing metal stent. Gastrointest Endosc 2016;83:460-1. [Crossref] [PubMed]
- Ge N, Sun S, Sun S, et al. Endoscopic ultrasound-assisted transmural cholecystoduodenostomy or cholecystogastrostomy as a bridge for per-oral cholecystoscopy therapy using double-flanged fully covered metal stent. BMC Gastroenterol 2016;16:9. [Crossref] [PubMed]
- Kumta NA, Lordello Passos M, Rodela Silva GL, et al. Endoscopic ultrasound-guided trans-mural gallbladder drainage with a lumen-apposing metal stent using an electrocautery enhanced delivery system. Endoscopy 2016;48:E327. [Crossref] [PubMed]
- Choi JH, Kim HW, Lee JC, et al. Percutaneous transhepatic versus EUS-guided gallbladder drainage for malignant cystic duct obstruction. Gastrointest Endosc 2017;85:357-64. [Crossref] [PubMed]
- Anderloni A, Attili F, Sferrazza A, et al. EUS-guided gallbladder drainage using a lumen-apposing self-expandable metal stent in patients with coagulopathy or anticoagulation therapy: a case series. Endosc Int Open 2017;5:E1100-3. [Crossref] [PubMed]
- Manta R, Zulli C, Zullo A, et al. Endoscopic ultrasound-guided gallbladder drainage for acute cholecystitis with a silicone-covered nitinol short bilaterally flared stent: a case series. Endosc Int Open 2017;5:E1111-5. [Crossref] [PubMed]
- Ahmed O, Ogura T, Eldahrouty A, et al. Endoscopic ultrasound-guided gallbladder drainage: Results of long-term follow-up. Saudi J Gastroenterol 2018;24:183-8. [Crossref] [PubMed]
- Peñas-Herrero I, Serna-Higuera C, Perez-Miranda M. Endoscopic ultrasound-guided gallbladder drainage for the management of acute cholecystitis (with video). J Hepatobiliary Pancreat Sci 2015;22:35-43. [Crossref] [PubMed]
- Widmer J, Singhal S, Gaidhane M, et al. Endoscopic ultrasound-guided endoluminal drainage of the gallbladder. Dig Endosc 2014;26:525-31. [Crossref] [PubMed]
- Hasan MK, Itoi T, Varadarajulu S. Endoscopic Management of Acute Cholecystitis. Gastrointest Endosc Clin N Am 2013;23:453-9. [Crossref] [PubMed]
- Lang GD, Fritz C, Bhat T, et al. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: comparison of efficacy and adverse event rates. Gastrointest Endosc 2018;87:150-7. [Crossref] [PubMed]
- James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound 2018;7:93-6. [PubMed]
- Kahaleh M. Training the next generation of advanced endoscopists in EUS-guided biliary and pancreatic drainage: learning from master endoscopists. Gastrointest Endosc 2013;78:638-41. [Crossref] [PubMed]
- Saumoy M, Novikov A, Kahaleh M. Long-term outcomes after EUS-guided gallbladder drainage. Endosc Ultrasound 2018;7:97-101. [PubMed]
Cite this article as: Posner H, Widmer J. EUS guided gallbladder drainage. Transl Gastroenterol Hepatol 2020;5:41.