
Acute-on-chronic liver failure in liver transplant candidates with non-alcoholic steatohepatitis
Introduction
Non-alcoholic fatty liver disease (NAFLD) and its aggressive form nonalcoholic steatohepatitis (NASH) has become the most common liver disease worldwide with an estimated global prevalence of 25% (1). The disease burden is attributed to obesity and metabolic syndrome and mirrors the rapidly rising global prevalence of obesity in both children and adults (2) as well as the upward trend of type 2 diabetes that occurs despite implementation of preventive measures (3). It has been estimated that approximately two-thirds of adults with obesity and 58% of diabetics worldwide have NAFLD (1). The NAFLD is forecasted to increase to 33.5% in 2030 in the USA with proportionally growing incidence of decompensated cirrhosis and hepatocellular carcinoma due to NASH (4). This will result in more patients with decompensated liver disease that will require hospitalizations and will eventually need liver transplantation (LT).
A subpopulation of patients with chronic liver disease and/or decompensated cirrhosis may develop acute-on-chronic liver failure (ACLF), a distinct syndrome characterized by rapid deterioration of liver function, development of one or more organ failures (OFs), and high short-term mortality (5). There is significant variability in definitions of ACLF with more than ten existing ones that have used different clinical, laboratory, and prognostic criteria, which largely restricts comparability between studies (6). Currently, there are three major definitions including the one of the European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) that is based on the results of a large, multicenter, prospective, observational study (CANONIC) (7); the recently updated consensus definition of ACLF of the Asian Pacific Association for the study of the Liver (APASL) (8); and the definition of the North American consortium for the study of end-stage liver disease (NACSELD) (9) (Table 1).
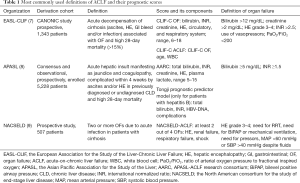
Full table
Precipitating factors and pathophysiology of ACLF
Triggers of decompensation
A major difference exists between the three main definitions regarding precipitating events. The most common precipitating events for EASL-CLIF and NACSELD are bacterial infections (7,9), alcohol use, and gastrointestinal bleeding (7), while the major triggering event for ACLF in APASL consensus is hepatic insult, such as hepatitis B reactivation, drug-induced liver injury, superimposed hepatitis A or E, or alcohol consumption (10). It is important to note that 43.6% of the patients in the CANONIC study did not have an identifiable precipitating event (7).
General concepts of ACLF pathophysiology
Current data suggest that the most important underlying pathogenic mechanism of ACLF is systemic inflammation (11). This hypothesis was initially supported by the results of the CANONIC study that showed patients with ACLF had elevated leucocyte count and C-reactive protein even if they did not have underlying infection or prior history of acute decompensation (7). When patients with decompensated cirrhosis were compared to healthy controls, systemic inflammation was more severe in those with ACLF compared to those without and its severity correlated with ACLF grade (11). Chronic inflammation in decompensated cirrhosis is attributed to translocation of pathogen-associated molecular patterns (PAMPs) that are recognized by different pattern recognition receptors: the membrane-bound toll-like receptors and the cytosolic nucleotide-binding oligomerization domain-like receptors, which leads to activation of inflammatory cascades and secretion of proinflammatory cytokines and reactive oxygen species (12). Systemic inflammation can exacerbate the preexistent systemic circulatory dysfunction and this may cause hypoperfusion and OFs leading to hepatic and extrahepatic cell damage and necrosis with resultant release of damage-associated molecular patterns (DAMPs), which stimulate the innate immune response and perpetuate acute inflammation (13). Liver injury further exacerbates systemic inflammation by cytokine/chemokine spillover to the blood and release of DAMPs.
Another important aspect of the pathogenesis of ACLF is cirrhosis-associated immune dysfunction. It results in altered homeostasis between systemic inflammation and hepatic immune defense. The latter is attributed to loss of surveillance and decreased liver synthetic function with lower production of albumin, complement, and acute phase proteins (14). While the proinflammatory phenotype is considered characteristic for the early phases of cirrhosis, the immunodeficient state is predominant in the later decompensated cirrhosis stages and ACLF. Moreover, genetic factors might be also playing a role as recent study identified two protective polymorphisms of interleukin-1 gene cluster that were associated with lower short-term mortality and lower susceptibility to ACLF (15).
Suggested ACLF pathophysiology in patients with NASH
The exact pathogenic factors contributing for progression of decompensated NASH cirrhosis to ACLF remain unclear. Given the majority of NASH patients also have obesity and type 2 diabetes mellitus (T2DM), these metabolic derangements are intricately interrelated and are likely to contribute for this progression. There is growing evidence on the pivotal role of gut-liver axis for the maintenance of metabolic homeostasis. It has been demonstrated that patients with obesity and NASH have a higher prevalence of small intestinal bacterial overgrowth (16). NASH has been associated with increased intestinal permeability (17). Patients with obesity and T2DM were noted to have significant gut dysbiosis with higher level of opportunistic pathogens such as Staphylococcus aureus, Escherichia coli, and Clostridium genus, while beneficial short chain fatty acid-producing bacteria were reduced (18). Moreover, animal data have shown that obesity and diabetes are related not only with translocation and elevated plasma levels of bacterial fragments such as lipopolysaccharides, but also of live commensal intestinal bacteria to blood and adipose tissue that causes persistent low-grade inflammation (19). All these factors might be contributing for development of ACLF in NASH patients.
ACLF in NASH patients
Prevalence and incidence
The prevalence of ACLF among patients with decompensated cirrhosis is estimated to range between 24% and 34% (7,9,20,21). In the CANONIC study, 10.8% of the hospitalized patients developed ACLF within 28 day (7) while 14% of stable outpatients with cirrhosis were diagnosed with ACLF within 1 year (22). These studies used EASL-CLIF diagnostic criteria to define ACLF. However, a recent retrospective study by Mahmud et al. noted significant disagreement between EASL and APASL criteria when both definitions were applied on the same population of 80,383 patients of the Veteran Health Administration (VA) system with compensated cirrhosis (23). The incidence of ACLF among NASH cirrhosis patients was estimated to be 3.4/1,000 (95% CI, 2.9–4.0) based on EASL-CLIF criteria and 18.6/1,000 (95% CI, 17.4–19.9) based on APASL criteria. When stratified by etiology of liver disease, patients with NASH and those with hepatitis C had the lowest incidence rate, but the highest 28- and 90-day mortality (51% and 73.5% by APASL and 45.2% and 59.2% by EASL definition, respectively). Infection was the most common precipitant factor for NASH patients with ACLF based on EASL definition. Among those with ACLF grade 3 (ACLF-3), patients with NASH and hepatitis C etiology had the highest rates of circulatory failure although kidney failure remained the leading OF.
Hospitalization rates and clinical characteristics of ACLF in patients with NASH
A recent population-based analysis that used the National Inpatient Sample (NIS) between 2006 and 2014 noted that NASH was the fastest growing liver disease etiology of all ACLF hospitalizations with an increase of 63% over the study period (3.5% in 2006–2008 to 5.7% in 2012–2014, P<0.001) (24). There was no change in ACLF hospitalizations due to viral liver diseases, but the increase for alcohol-related cirrhosis was 28%. The frequency of NASH-related ACLF admissions also showed the most rapid rise: from 12% in 2006–2008 to 55% in 2012–2014. In corroboration with the previous study (23), infection was the most common precipitating event in ACLF due to NASH cirrhosis (80%) (24). Sepsis and septic shock were also the most frequent event in NASH patients (67% and 51%, respectively). However, although patients with ACLF due to NASH cirrhosis required the longest hospitalization and had the highest hospital charges among all etiologies, they had reduced odds of inpatient mortality. Similarly to the study by Mahmud et al. (23), circulatory failure was the most common OF in patients with NASH-related ACLF (74%) (24).
Interestingly, when non-obese patients with ACLF were compared to those with obesity class 1–2, and to obesity class 3 in a population of patients with different etiologies of liver disease, it was noted that renal failure was the most common OF that increased in prevalence along with obesity class while circulatory failure was inversely correlated with worsening obesity (25). Bacterial infections were the major decompensating event in ACLF patients with obesity class 3 (59.5%) while ascites and variceal bleeding were more common in the non-obese group.
On balance, ACLF in patients with NASH cirrhosis has been increasing based on hospitalization data. Infections are the most common precipitant factor with frequent sepsis and septic shock in this cohort. Circulatory failure is the leading cause of OF in patients with NASH, but it was not increased in those with morbid obesity and different other liver etiologies. These observations suggest that NASH might have independent role for circulatory dysfunction, which on the other side has been shown to be a predictor of ACLF development (22). When compared to other etiologies, ACLF due to NASH had lower inpatient mortality (24), but higher 28- and 90-day mortality (23). The increase in short-term mortality could be associated with the higher rate of infections in this population as a study based on the CANONIC database has demonstrated that bacterial infections were independent predictors of 90-day mortality in patients with ACLF grade 1 (ACLF-1) and grade 2 (ACLF-2) (26).
Natural history, prognosis, and mortality
Predictors of NASH progression to ACLF
A long-term follow up study has shown that 25% of patients with NASH progressed to cirrhosis over nine years with 10% developing decompensating events over 13 years (27). Obesity and T2DM, frequently associated with NAFLD, also contribute for liver disease progression. Clinical studies have demonstrated that obesity, an independent predictor of cirrhosis decompensation (28), is associated with infections in decompensated cirrhosis (29), and a higher in-hospital mortality in cirrhotic patients with sepsis (30). Importantly, morbid obesity [hazard ratio (HR) 1.21, 95% CI, 1.07–1.37] and diabetes (HR 1.15; 95% CI, 1.06–1.23) were linked to increased risk of dropout of NASH patients on the LT list (31).
Prognostic scores and predictors of severity of ACLF in patients with all etiologies
Different scores and prognostic markers have been used in various studies that included patients with ACLF (Table 1). Based on a derivation cohort of the CANONIC study, a CLIF-C acute decompensation score has been created that predicts 3- and 12-month mortality in hospitalized patients with cirrhosis, but without ACLF (32). This score performs better than Child-Pugh, Model for End-Stage Liver Disease (MELD), and MELD-Sodium (MELD-Na) scores. It includes age, serum sodium, white blood cell (WBC) count, serum creatinine, and international normalized ratio (INR) and ranges between 0 and 100 points with score 60 identifying a high-risk group of patients with 90-day mortality exceeding 30% and close to the 90-day mortality of ACLF-1 group in the CANONIC study (7). Among prospectively followed ACLF patients, ACLF grade at days 3–7 predicted mortality better than ACLF grade at diagnosis (33). The best independent predictors of severe early course were CLIF-C ACLF score (odds ratio [OR] 1.11; 95% CI, 1.07–1.15) and presence of liver failure defined as total bilirubin 12 mg/dL (OR 2.82; 95% CI, 1.72–4.63). This study also reported that patients with ACLF-3 at day 3–7 who had 4 or more OFs and CLIF-C ACLF score >64 carried a very poor prognosis with 100% mortality at three months. When compared to other scores, CLIF-C ACLF also best predicted 28-day mortality with a threshold of 370 points at 48 hours portending 100% mortality (34). Future studies should validate the applicability and utility of these scores in patients with NASH cirrhosis and ACLF.
Short-term mortality in NASH patients with ACLF
The estimated short-term mortality was higher in NASH patients who develop ACLF when compared to that of the overall population in the CANONIC study (7) and a recent study that included large cohort of VA patients (21) (Table 2). However, this comparison is approximate as these populations might have consisted of patients with different proportions of ACLF severity based on ACLF grade and type of OF, age, gender, ethnicity, and comorbidities.
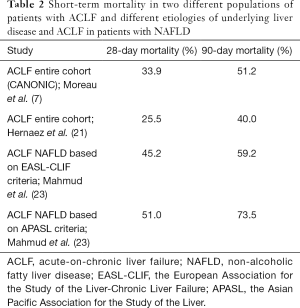
Full table
Role of LT
Short-term mortality of the waitlisted patients with ACLF and all etiologies
Growing evidence suggests that patients with ACLF-3 should be considered early for LT (35) and perhaps prioritized (36). If properly selected and transplanted early, these patients have post-LT outcomes comparable to the general population (37). A recent analysis of the United Network for Organ Sharing (UNOS) database demonstrated that patients with ACLF-3 and MELD-Na <25 were more likely to die or to be removed from the LT list when compared to the rest of ACLF patients (35). Another analysis of the same data registry showed that the probability of survival for more than 30 days on the transplant list is inversely related to the number of OFs with 80% removed with two OFs and 92–98% removed with three or more OFs, the latest having median time to death of 6–10 days (37). Moreover, a study that compared short-term waitlist mortality and delisting between patients with acute liver failure who were listed as status 1a and registrants with ACLF-3 at listing noted that patients with ACLF-3 had higher risk of 14-day mortality across all MELD-Na subgroups (36). Both groups in this study had numerically similar 1-year post-LT survival. The authors suggested that alternative scores for evaluation might be needed in these patients as MELD-Na does not reflect OFs.
Post-LT outcomes and predictors of survival of patients with ACLF and all etiologies
Previous studies have shown one-year post-LT survival in patient with ACLF ranging between 46% and 87% (33,38,39). However, these results are based on very small sample sizes, different ACLF definitions, and varying severity of ACLF at inclusion and at the time of transplant. One retrospective and two UNOS-based studies assessed outcomes in patients with ACLF-3 (35,37,40). One-year mortality in this population was 81-84%. Although respiratory failure requiring mechanical ventilation at the time of LT was identified as an independent risk factor in one of those studies (35), there was no association in another study (37). Other independent negative predictors for survival were high-risk donors (35,37), hepatitis C infection etiology or hepatocellular carcinoma, and the number of OFs (37), while LT within 30 days of listing was a positive predictor (35). NASH was not associated with survival differences in both studies (35,37).
Important considerations in the pre-transplant evaluation of patients with NASH and ACLF
Considering the strong evidence that NAFLD is associated with incident and prevalent hypertension and coronary artery disease and that it has high cardiovascular disease-related mortality (41), patients with NASH, who are diagnosed with ACLF should be considered early for transplant evaluation. Moreover, prompt cardiovascular assessment should be performed before these patients develop circulatory failure since this type of OF is more common in patients with NASH-related ACLF (23,24).
Therapeutic options
General principles of treatment of patients with NASH and ACLF
At present, there are no specific therapies for ACLF. The mainstay of treatment consists of identification and treatment of precipitating factors and management of OF (42). NASH patients should undergo prompt screening for infection and sepsis followed by immediate initiation of antibiotic therapy as infections were noted to be the most common precipitating event in this cohort (23,24). It is important when choosing empiric antibiotic therapy to take into consideration that nosocomial infections and infections caused by gram-positive bacteria or multi-drug resistant organisms are more frequent among ACLF patients (26). Fungal infections should be considered when there is no response to initial antibiotic therapy and/or the patients have concomitant T2DM. Adequate initial antibiotic therapy has been associated with better evolution and lower short-term mortality (26).
Role of artificial liver support systems in ACLF treatment
Artificial liver support systems are based on the concept of albumin dialysis and have been investigated as a potential option for bridging ACLF patients to LT. The best studied ones are the Molecular Adsorbent Recirculating System (MARS) and the Fractionated Plasma Separation and Adsorption system (Prometheus). The effect of these systems derives from their ability to remove albumin-bound toxins, nitric oxide, and pro-inflammatory cytokines (43). However, multicenter randomized controlled trials (RCTs) that investigated MARS vs. standard therapy (44) and Prometheus vs. standard therapy (45) failed to demonstrate benefit on 28- and 90-day survival. Another retrospective study showed MARS reduced early mortality at day 7 and 14 and suggested that the system might be more beneficial in patients with 2 or more OFs (46). The limitations of these studies were heterogeneity of patients’ selection and use of different ACLF definitions. A recent meta-analysis based on seven RCTs and 3 observational studies demonstrated a beneficial effect of artificial liver support systems on short-term survival in ACLF patients (47). Future studies of well-selected patients with ACLF should assess the utility of these support systems on survival of patients with NASH and assess whether their effect differs among patients with various etiologies of liver disease.
Role of granulocyte colony-stimulating factor (G-CSF) and cell therapy in ACLF
Other tested therapeutic options for ACLF are G-CSF administration and stem cell transplantation. The suggested beneficial effect of G-CSF is based on stem cell mobilization that could boost liver regeneration and on an immune modulation effect that can ameliorate immune dysfunction associated with ACLF. These potential effects may reduce the risk of infections (48). Two small RCTs (49,50) and a meta-analysis (51) have shown improvement in short-term survival, MELD, and Child-Pugh score, but due to the differences in ACLF definitions, population included, and variable doses and duration of G-CSF, it is difficult to make any firm conclusions on the efficacy of this treatment.
Animal data have demonstrated that transfusion of mesenchymal stem cells (MSCs) in hepatic failure may improve liver function, reduce inflammation, and reverse fibrosis (52). MSCs can be isolated from umbilical cord, bone marrow, or adipose tissue. The exact mechanism of MSCs for liver regeneration remains controversial, but it is considered they can differentiate into hepatocyte-like cells and integrate into the liver tissue, in addition to immunomodulatory effects and secretion of anti-inflammatory cytokines (53). To date, only one small open-label trial assessed the effect of umbilical cord-derived MSCs in patients with ACLF due to hepatitis B infection (54). It showed improved survival at 90 days and decreased MELD with no significant side effects. Currently, a phase 2 trial is investigating the safety of different doses of human liver-derived progenitor cells (HepaStem®, HHALPCs, Promethera Biosciences, Belgium) in patients with ACLF or with acute decompensation who are at risk for ACLF (NCT02946554). Another ongoing open-label trial has the aim to assess the safety and tolerability of HepaStem in cirrhotic and precirrhotic patients with NASH, but acute decompensation and ACLF are exclusion criteria (NCT03963921). The results of these trials are greatly awaited.
Conclusions
Despite the growing burden on NASH worldwide, there is a paucity of data that have assessed ACLF progression and outcomes in this population. Future studies should focus on the pathophysiology, clinical course, optimal LT evaluation, the impact of metabolic and cardiovascular comorbidities on LT candidacy and post-LT outcomes in NASH patients who develop ACLF.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Younossi Z, Tacke F, Arrese M, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019;69:2672-82. [Crossref] [PubMed]
- GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017;377:13-27.
- Bullard KM, Cowie CC, Lessem SE, et al. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:359-61. [Crossref] [PubMed]
- Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123-33. [Crossref] [PubMed]
- Arroyo V, Moreau R, Kamath PS, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers 2016;2:16041. [Crossref] [PubMed]
- Wlodzimirow KA, Eslami S, Abu-Hanna A, et al. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int 2013;33:40-52. [Crossref] [PubMed]
- Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426-37, 1437.e1-9.
- Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int 2019;13:353-90. [Crossref] [PubMed]
- Bajaj JS, O'Leary JG, Reddy KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250-6. [Crossref] [PubMed]
- Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int 2019;13:353-90. [Crossref] [PubMed]
- Clària J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 2016;64:1249-64. [Crossref] [PubMed]
- Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63:1272-84. [Crossref] [PubMed]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 2008;8:279-89. [Crossref] [PubMed]
- Irvine KM, Ratnasekera I, Powell EE, et al. Causes and Consequences of Innate Immune Dysfunction in Cirrhosis. Front Immunol 2019;10:293. [Crossref] [PubMed]
- Alcaraz-Quiles J, Titos E, Casulleras M, et al. Polymorphisms in the IL-1 gene cluster influence systemic inflammation in patients at risk for acute-on-chronic liver failure. Hepatology 2017;65:202-16. [Crossref] [PubMed]
- Sabaté JM, Jouet P, Harnois F, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg 2008;18:371-7. [Crossref] [PubMed]
- Luther J, Garber JJ, Khalili H, et al. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol Gastroenterol Hepatol 2015;1:222-32. [Crossref] [PubMed]
- Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med 2013;34:39-58. [Crossref] [PubMed]
- Amar J, Chabo C, Waget A, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 2011;3:559-72. [Crossref] [PubMed]
- Li H, Chen LY, Zhang NN, et al. Characteristics, Diagnosis and Prognosis of Acute-on-Chronic Liver Failure in Cirrhosis Associated to Hepatitis B. Sci Rep 2016;6:25487. [Crossref] [PubMed]
- Hernaez R, Kramer JR, Liu Y, et al. Prevalence and short-term mortality of acute-on-chronic liver failure: A national cohort study from the USA. J Hepatol 2019;70:639-47. [Crossref] [PubMed]
- Piano S, Tonon M, Vettore E, et al. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol 2017;67:1177-84. [Crossref] [PubMed]
- Mahmud N, Kaplan DE, Taddei TH, et al. Incidence and Mortality of Acute-on-Chronic Liver Failure Using Two Definitions in Patients with Compensated Cirrhosis. Hepatology 2019;69:2150-63. [Crossref] [PubMed]
- Axley P, Ahmed Z, Arora S, et al. NASH Is the Most Rapidly Growing Etiology for Acute-on-Chronic Liver Failure-Related Hospitalization and Disease Burden in the United States: A Population-Based Study. Liver Transpl 2019;25:695-705. [Crossref] [PubMed]
- Sundaram V, Jalan R, Ahn JC, et al. Class III obesity is a risk factor for the development of acute-on-chronic liver failure in patients with decompensated cirrhosis. J Hepatol 2018;69:617-25. [Crossref] [PubMed]
- Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 2018;67:1870-80. [Crossref] [PubMed]
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865-73. [Crossref] [PubMed]
- Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology 2011;54:555-61. [Crossref] [PubMed]
- Sundaram V, Kaung A, Rajaram A, et al. Obesity is independently associated with infection in hospitalised patients with end-stage liver disease. Aliment Pharmacol Ther 2015;42:1271-80. [Crossref] [PubMed]
- Kok B, Karvellas CJ, Abraldes JG, et al. The impact of obesity in cirrhotic patients with septic shock: A retrospective cohort study. Liver Int 2018;38:1230-41. [Crossref] [PubMed]
- Kardashian AA, Dodge JL, Roberts J, et al. Weighing the risks: Morbid obesity and diabetes are associated with increased risk of death on the liver transplant waiting list. Liver Int 2018;38:553-63. [Crossref] [PubMed]
- Jalan R, Pavesi M, Saliba F, et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol 2015;62:831-40. [Crossref] [PubMed]
- Gustot T, Fernandez J, Garcia E, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243-52. [Crossref] [PubMed]
- Engelmann C, Thomsen KL, Zakeri N, et al. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care 2018;22:254. [Crossref] [PubMed]
- Sundaram V, Jalan R, Wu T, et al. Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology 2019;156:1381-91.e3. [Crossref] [PubMed]
- Sundaram V, Shah P, Wong RJ, et al. Patients With Acute on Chronic Liver Failure Grade 3 Have Greater 14-Day Waitlist Mortality Than Status-1a Patients. Hepatology 2019;70:334-45. [PubMed]
- Thuluvath PJ, Thuluvath AJ, Hanish S, et al. Liver transplantation in patients with multiple organ failures: Feasibility and outcomes. J Hepatol 2018;69:1047-56. [Crossref] [PubMed]
- Umgelter A, Lange K, Kornberg A, et al. Orthotopic liver transplantation in critically ill cirrhotic patients with multi-organ failure: a single-center experience. Transplant Proc 2011;43:3762-8. [Crossref] [PubMed]
- Levesque E, Winter A, Noorah Z, et al. Impact of acute-on-chronic liver failure on 90-day mortality following a first liver transplantation. Liver Int 2017;37:684-93. [Crossref] [PubMed]
- Artru F, Louvet A, Ruiz I, et al. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol 2017;67:708-15. [Crossref] [PubMed]
- Wu S, Wu F, Ding Y, et al. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep 2016;6:33386. [Crossref] [PubMed]
- Nadim MK, Durand F, Kellum JA, et al. Management of the critically ill patient with cirrhosis: A multidisciplinary perspective. J Hepatol 2016;64:717-35. [Crossref] [PubMed]
- Guo LM, Liu JY, Xu DZ, et al. Application of Molecular Adsorbents Recirculating System to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int 2003;23 Suppl 3:16-20. [Crossref] [PubMed]
- Bañares R, Nevens F, Larsen FS, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology 2013;57:1153-62. [Crossref] [PubMed]
- Kribben A, Gerken G, Haag S, et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 2012;142:782-9.e3. [Crossref] [PubMed]
- Gerth HU, Pohlen M, Tholking G, et al. Molecular Adsorbent Recirculating System Can Reduce Short-Term Mortality Among Patients With Acute-on-Chronic Liver Failure-A Retrospective Analysis. Crit Care Med 2017;45:1616-24. [Crossref] [PubMed]
- Shen Y, Wang XL, Wang B, et al. Survival Benefits With Artificial Liver Support System for Acute-on-Chronic Liver Failure: A Time Series-Based Meta-Analysis. Medicine (Baltimore) 2016;95:e2506. [Crossref] [PubMed]
- Khanam A, Trehanpati N, Garg V, et al. Altered frequencies of dendritic cells and IFN-gamma-secreting T cells with granulocyte colony-stimulating factor (G-CSF) therapy in acute-on- chronic liver failure. Liver Int 2014;34:505-13. [Crossref] [PubMed]
- Garg V, Garg H, Khan A, et al. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 2012;142:505-12.e1. [Crossref] [PubMed]
- Duan XZ, Liu FF, Tong JJ, et al. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol 2013;19:1104-10. [Crossref] [PubMed]
- Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ, et al. Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Ann Hepatol 2015;14:631-41. [Crossref] [PubMed]
- Hwang S, Hong HN, Kim HS, et al. Hepatogenic differentiation of mesenchymal stem cells in a rat model of thioacetamide-induced liver cirrhosis. Cell Biol Int 2012;36:279-88. [Crossref] [PubMed]
- Meier RP, Muller YD, Morel P, et al. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res 2013;11:1348-64. [Crossref] [PubMed]
- Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med 2012;1:725-31. [Crossref] [PubMed]
Cite this article as: Doycheva I, Thuluvath PJ. Acute-on-chronic liver failure in liver transplant candidates with non-alcoholic steatohepatitis. Transl Gastroenterol Hepatol 2020;5:38.


