
Endocytoscopy: technology and clinical application in upper gastrointestinal tract
Introduction
With the goal of optimizing the detection and diagnosis of early-stage cancer in the gastrointestinal (GI) tract, advancement of endoscopic imaging technologies has been in progress in the recent years, despite histopathological examination remaining as the gold standard (1,2). More commonly utilized are magnifying endoscopy with narrow-band imaging (NBI) and chromoendoscopy, whereas others, such as confocal endomicroscopy and endocytoscopy, are seldom applied (3).
Endocytoscopy (EC) is a novel ultra-high magnification endoscopic technique designed to provide excellent in-vivo assessment of lesions found in the GI tract. With the use of intraprocedural stains, EC allows microscopic visualization of the GI mucosal surface (4). Following the advent of first-generation EC in 2003, several enhancements have been accomplished, paving the way for the development of the cutting-edge fourth-generation endocytoscopes (5).
The main scope of this review article is to offer a closer look at the latest EC technology and its clinical application in the upper GI tract, especially in the esophagus and stomach, as well as to introduce readers to our simplified and up-to-date EC classification, specifically developed for the esophagus and stomach, for the in-vivo assessment and diagnosis of esophageal and gastric lesions.
Development of EC
Kumagai et al. published a detailed description of the differences and modifications between the four generations of endocytoscopes that have appeared since 2003 (5,6), and has been summarized in Table 1. A probe-type first-generation EC with an outer diameter of 3.2 mm was developed in 2003 which allowed a fixed-focus of 1,125× (XEC120U; Olympus Medical Systems Corp., Tokyo, Japan). The small diameter allowed the endocytoscope to pass through a wider channel therapeutic endoscope. This was followed by the second-generation EC in 2005 equipped with a double integrated-type lens, fixed focus of 450×, and an 11.6 mm outer diameter (GIF-Y0001; Olympus Medical Systems Corp., Tokyo, Japan). Third-generation EC appeared in 2009 owning a single integrated-type lens and a 10.7 mm outer diameter (GIF-Y0002; Olympus Medical Systems Corp., Tokyo, Japan). In comparison from the fixed-focus of the previous two generations of endocytoscopes, this third-generation has a continuous increase in magnification of up to 380× allowing a significant improvement in the visualization of cells and cellular structure. However, there were still challenges in good evaluation of the nuclei. The latest endocytoscope is the fourth-generation, which first materialized in 2015 and is now commercially available. This fourth-generation endocytoscope has a single integrated-type lens reflection, up to 500× continuous zoom-focus magnification, observation range of 570 μm × 500 μm, and an outer diameter of 9.7 mm (GIF-H290EC; Olympus Medical Systems Corp., Tokyo, Japan) which is smaller than the standard endoscope used for screening endoscopic examinations. This allows acquisition of high-definition resolution EC images.

Full table
Methods
Procedure
All EC examinations are performed after white-light endoscopy and NBI, under intravenous sedation. Depending on the technical skill of the endoscopist, the average time to perform EC may take anywhere from 10 to 20 minutes. To date, the endocytoscope being utilized is the fourth-generation. This particular endocytoscope can likewise function as a standard screening endoscope and carry on with a full magnifying endoscopic examination when desired. The use of a distal attachment, a black silicone cap (Distal Hood MAJ-1989; Olympus Medical Systems Corp., Tokyo, Japan), secured in an oblique approach, facilitates the lens to come into contact with the mucosal surface (7).
CM double staining
One critical factor in acquiring good and assessable EC images is an appropriate staining solution and method. It is not a surprise that different staining solutions and methods have emerged in various literatures in the past years. So far, based on available data, three dyes with varying concentrations have been accounted for as the most utilized ones: toluidine blue (TB), methylene blue (MB), and crystal violet (CV). The issue remains which dye is the most suitable for EC, to which a number of studies have sought to provide answers. For instance, the use of 1% MB on squamous cell dysplasia and carcinoma, and 1% TB for intestinal type metaplasia has been previously recommended (8). In a more recent study by Goda et al., normal duodenal villi and superficial non-ampullary duodenal epithelial tumors (SNADETs) were assessed by EC using the three most common staining solutions. Their study suggested that 0.5% TB and 1% MB are the most suitable staining solutions for normal duodenal villi and SNADETs (9).
In our institution, a mixture of 10 cc 0.05% CV and 1 cc 1% MB, which we refer to as CM double staining, is used for EC assessment of both esophageal and gastric lesions. We use 1 cc of this mixture with 9 cc of air aspirated in separate 10 cc syringes and spray multiple times through the scope channel, with an interval of 15 to 30 seconds, until a satisfactory staining is achieved (Figure 1) (7). This CM double staining method, first developed in 2010 (10) and has been then applied in subsequent studies (11,12), produces a staining pattern resembling the traditional hematoxylin-eosin stain used in conventional microscopy.
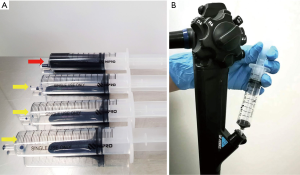
Cell nuclei are clearly stained with MB, whereas CV stains the cytoplasm, thus facilitating clear, detailed, and more rapid identification of the glandular structure (11). The use of MB alone is, in fact, not infrequent. Rather, it is a wildly used staining method in several studies, one of which was by Fujishiro et al. (13). In this study, iodine was initially used to assess suspected esophageal squamous cell carcinoma, followed by EC using 10 cc of 1% MB alone. Clear EC images were not obtained in 40% of the cases which they attributed to the prior use of iodine. Since iodine can cause esophageal mucosal damage, they have speculated that it may have had an effect on the uptake of MB by the cells. However, Minami et al. have indicated that using MB alone creates a darker staining, making it challenging to obtain a good quality image and clearly identify the cellular structures (12). Although there were reports of potential risk of DNA damage when using higher concentrations of MB alone in previous literatures (14-17), diluting it with CV decreases this risk and seems to provide a better image visualization.
Irrespective of the differences between staining solutions and methods applied, the major goal remains to be, without a doubt, the acquisition of high-quality assessable EC images.
EC classification
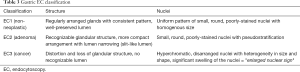
In 2011, a novel EC classification for the diagnosis of colorectal lesions has been published by Kudo et al. (18). Both structural and cellular atypia (lumen morphology, nuclear changes) were the main focus of this particular novel EC classification. By assessing these factors, EC1a and EC1b are identified as non-neoplastic while EC2, EC3a, and EC3b are considered as neoplastic lesions.
Based on an adaptation of this novel classification, our group has developed a simplified and up-to-date three-tier EC classification specific for the diagnosis of esophageal and gastric lesions. In principle, we identify and divide the lesions into non-neoplastic, borderline, and cancer, and classify them as EC1, EC2, and EC3, respectively. Similar to the colorectal EC classification, our main focus were cellular arrangement and morphology, and nuclear structure. Based on conventional histopathologic findings, we identify non-neoplastic lesions as those with regular cellular arrangement and uniform pattern of small rounded nuclei. Borderline lesions are those presenting with changes in the cellular density, morphology or arrangement, however, the nucleus remains small and with regular shape and size or may be mildly enlarged. Neoplastic lesions, on the other hand, are those with irregular cellular arrangement and morphology. Changes in the nucleus that we observe are heterogeneity in shape and size, hyperchromasia, and significant swelling. We summarize the EC classification for both esophageal (Figure 2) and gastric (Figure 3) lesions on Tables 2,3.
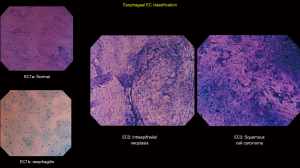
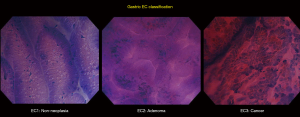
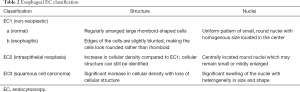
Full table
Full table
Esophageal EC
The squamous epithelium of the esophageal mucosa is more suitable for staining and EC assessment (19), thereby more esophageal EC studies have been conducted. Factors considered in previous reports in making an esophageal EC assessment included cellular arrangement and density, cellular size and shape, nuclear size and shape, and the nucleus: cytoplasm (N:C) ratio (13,20). Normal esophageal mucosa appears to have regular arrangement of large rhomboid-shaped cells. The nucleus, located in the center of the cell, appears to be small and uniformly sized. In contrast, malignant lesions show an apparent increase in the cellular and nuclear density. The cells are irregularly arranged, and the N:C ratio is increased. In an ex-vivo study by Kodashima et al., comparison of the nuclear density between normal squamous epithelium and that of squamous cell carcinoma was done which revealed a significantly increased nuclear density in the latter (21). In addition, another multicenter ex-vivo study by Fujishiro et al. supported the aforementioned study depicting that an increase in the nuclear density is indeed observed in malignant lesions (13).
Application of EC on Barrett’s esophagus has also been previously reported, although the results for this appear to be controversial. In this study by Pohl et al. in 2007, 49% of the images were not adequately assessed by EC (22). It is tempting to speculate that the use of second-generation EC with a lower magnification at the time of their study could have been a contributing factor which may have potentially affected the results. In 2013, Eleftheriadis et al. made a first account describing in-vivo high quality images of squamous cell islands as round-shaped cells with heterogeneously-shaped small nuclei within regular Barrett’s epithelium by using third-generation EC (23). In this study, it was concluded that EC seems to be promising in the evaluation of Barrett’s mucosa compared to results from previous studies. In the same year, a pilot ex-vivo study by Tomizawa et al. was conducted, investigating the diagnostic performance of EC in Barrett’s esophagus by using their own Barrett’s EC classification system (24). Diagnostic accuracy of >90% for experts and >80% for non-experts, as well as an excellent inter-observer agreement of >0.85 were reported in this study. Indeed, additional future studies on this topic is desired to validate the results on the real role and ability of EC in diagnosing Barrett’s esophagus.
Different esophageal EC classifications have appeared in the past years. In 2006, Inoue et al. have described in their pilot study an esophageal EC classification with five grades based on endocytoscopic atypia (ECA) (25) in concordance with the Vienna classification. Comparing this five-grade ECA classification to histopathology yielded an accuracy of 82% in their study. This was followed by another EC classification by Kumagai et al. in conjunction with iodine staining (26). Using the third-generation EC, they achieved a sensitivity for malignancy of 94.9% (27). However, the specificity was 46.7% which they attributed to the low magnification power of the third-generation EC. At present, our group have simplified the previous five-grade ECA classification into a three-tier EC classification, described in Table 2.
Gastric EC
In comparison with esophageal EC, there are a smaller number of EC studies examining gastric lesions due to the increased mucus secretory function of the stomach (28) as a result of the absence of intestinal epithelium and its absorptive function (29). This abundance of mucus poses a challenge in achieving a satisfactory staining and obtaining high-quality EC images. To address this, several studies, one of which was by Chiu et al. (30), have suggested to optimize the staining technique to enhance the quality of EC images. Although they were able to demonstrate a good diagnostic performance of EC in recognizing goblet cells for the diagnosis of gastric intestinal metaplasia (IM), the image quality they obtained were not satisfactory. As we have described in our previous study (7), we tackle this “poor staining” issue by applying a water-based mixture containing the mucolytic pronase and anti-foaming agent dimethicone prior to the procedure along with careful low-flow water-jet assisted mucosal rinsing prior to CM double staining multiple times. Between these multiple stainings, an interval of approximately 15 to 30 seconds is observed to ensure adequate dye uptake. By performing this method in our previous study, we were able to attain high-quality images in over 80% of the cases (7).
Gastric EC has been previously reported to be carried out in various circumstances, ranging from assessment of non-neoplastic changes to evaluation of suspected malignant lesions. Non-neoplastic changes using EC have been described by Sato et al. (31). Normal gastric mucosa, as seen on EC, appears to have regular glands, smooth surfaces and soft edges, well-preserved lumen, and small uniformly sized rounded nuclei with poor staining. There are no infiltrating cells, necrotic tissue, or debris. Well-stained crypts and presence of infiltrating cells and debris can be observed on EC images of chronic gastritis. Glands are still regular in shape and size, and with preserved lumen. Hyperplastic polyps have been described as having wider, star-like lumen, with small, regular nuclei (32). A pilot ex-vivo study using EC to observe a living microorganism, H. pylori, was published by Kimura et al., capturing a video of moving and spinning rod-shaped bacteria akin to typical H. pylori findings in conventional microscopy (33). Assessment of signet ring cell carcinoma (SRCC) of the stomach using EC has also been previously reported by Fasoli et al. (34). In their article, absence of a distinct glandular structure and the presence of a peripherally located nucleus surrounded by a cytoplasmic halo has been observed in EC assessment of SRCC, which corresponded to the typical findings of SRCC in conventional histopathology. Isomoto et al., in 2013, presented the first study of applying EC in gastric lymphomas, revealing an exclusive mucosal aggregation of cellular structures as EC findings in all gastric lymphoma cases except for one case of mucosa-associated lymphoid tissue (MALT) (35). Clear recognition and identification of goblet cells have been the characteristic EC finding of gastric IM as described by Chiu et al. in 2014 (30).
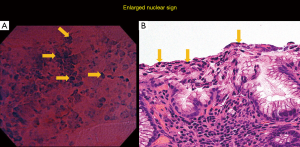
Adding to this information, our group has also reported a major finding in our previous study, the identification of the newly-recognized “enlarged nuclear sign” (ENS) (Figure 4) (7). ENS is a hyperchromatic nucleus that is large enough to give the impression of “taking over” the entire cell surface (7). ENS can be disarranged and with heterogenous shape (large and elongated, large with rough edges). Traditionally, the N:C ratio has been used in histopathologic and endocytoscopic evaluation (31,36). However, this characteristic ENS finding being detected in EC images of well-differentiated gastric adenocarcinomas in our previous study urged our group to utilize it as a distinct feature.
Current and future challenges
The innovative field of magnifying endoscopy has been expanded over the years with various cutting-edge technologies. EC has been gradually emerging in the recent years as an efficient ultra-high magnification endoscopic imaging technique most especially in Japan. Due to the physical structure of the latest fourth-generation endocytoscopy, it has become possible to use the same scope for screening endoscopy, as well as a full magnifying endoscopic examination if warranted. However, the limited availability of the endocytoscopes, which are only available in a small number of centers worldwide, serves as an issue on why EC is less known and less utilized outside Japan. Apart from that, the subject on performing an adequate staining method and using the appropriate staining solution to acquire good quality and assessable images still remain a topic of discussion among endoscopists. Hence, our group has aimed to report the method we utilize in our institution to obtain good quality EC images in our efforts of addressing this issue.
Another challenge that needs to be addressed is the standardization of the classification to be applied in making in-vivo diagnosis of GI lesions. In our attempt to tackle this and to establish an easier and more usable classification, we developed our simplified and up-to-date three-tier EC classification for both esophageal and gastric lesions based on an adaptation from the original colorectal EC classification. Further studies utilizing these two updated EC classifications are necessary to assess and confirm its reproducibility and its potential of becoming the universal EC classification.
Until recently, a known major limitation of EC is its inability to visualize beyond the superficial epithelial layer. Although this limitation seems to be addressed in the lower GI tract (37), the ability of EC to assess the depth of invasion of an upper GI tract lesion remains to be elucidated.
Overall, it seems that EC has proven to have a good diagnostic accuracy, offering to aid in the in-vivo diagnosis of esophageal and gastric lesions, and deserving further evaluation in forthcoming studies. Perhaps, in the future, EC could revolutionize the field of in-vivo endoscopic GI cancer diagnosis, bringing us a step closer to the keen desire of every endoscopist, the so-called optical biopsy.
Acknowledgments
None.
Footnote
Conflicts of Interest: H Inoue is an advisor of Olympus Corporation and Top Corporation. He has also received educational grants from Olympus Corp., and Takeda Pharmaceutical Co. Other authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Tsurudome I, Miyahara R, Funasaka K, et al. In vivo histological diagnosis for gastric cancer using endocytoscopy. World J Gastroenterol 2017;23:6894-901. [Crossref] [PubMed]
- Inoue H, Kudo SE, Shiokawa A. Technology insight: Laser-scanning confocal microscopy and endocytoscopy for cellular observation of the gastrointestinal tract. Nat Clin Pract Gastroenterol Hepatol 2005;2:31-7. [Crossref] [PubMed]
- Sumiyama K. Past and current trends in endoscopic diagnosis for early stage gastric cancer in Japan. Gastric Cancer 2017;20:20-7. [Crossref] [PubMed]
- Kaise M, Ohkura Y, Iizuka T, et al. Endocytoscopy is a promising modality with high diagnostic accuracy for gastric cancer. Endoscopy 2015;47:19-25. [PubMed]
- Kumagai Y, Kawada K, Takubo K, et al. Ultra-high magnification endoscopy (endocytoscopy system) for examination of esophageal lesions. Gastroenterol Endosc 2017;59:207-18.
- Kumagai Y, Takubo K, Kawada K, et al. A newly developed continuous zoom-focus endocytoscope. Endoscopy 2017;49:176-80. [PubMed]
- Abad MRA, Inoue H, Ikeda H, et al. Utilizing fourth-generation endocytoscopy and the “enlarged nuclear sign” for in-vivo diagnosis of early gastric cancer. Endosc Int Open 2019;7:E1002-7. [Crossref] [PubMed]
- Pirogov SS, Sokolov VV, Kaprin AD, et al. Endocytoscopy-novel endoscopic diagnostics approach: principles and procedure. Eksp Klin Gastroenterol 2015.12-21. [PubMed]
- Goda K, Dobashi A, Yoshimura N, et al. Dye solution optimizing staining conditions for in vivo endocytoscopy for normal villi and superficial epithelial tumors in the duodenum. Ann Gastroenterol 2019;32:378-86. [PubMed]
- Inoue H, Yokoyama A, Kudo SE. Ultrahigh magnifying endoscopy: development of CM double staining for endocytoscopy and its safety. Nihon Rinsho 2010;68:1247-52. [PubMed]
- Ichimasa K, Kudo SE, Mori Y, et al. Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: an in vivo prospective pilot study. Dig Endosc 2014;26:403-8. [Crossref] [PubMed]
- Minami H, Inoue H, Yokoyama A, et al. Recent advancement of observing living cells in the esophagus using CM double staining: endocytoscopic atypia classification. Dis Esophagus 2012;25:235-41. [Crossref] [PubMed]
- Fujishiro M, Takubo K, Sato Y, et al. Potential and present limitation of endocytoscopy in the diagnosis of esophageal squamous-cell carcinoma: a multicenter ex vivo pilot study. Gastrointest Endosc 2007;66:551-5. [Crossref] [PubMed]
- Repici A, Ciscato C, Wallace M, et al. Evaluation of genotoxicity related to oral methylene blue chromoendoscopy. Endoscopy 2018;50:1027-32. [Crossref] [PubMed]
- Di Stefano AFD, Radicioni MM, Vaccani A, et al. Methylene blue MMX(R) tablets for chromoendoscopy. Bioavailability, colon staining and safety in healthy volunteers undergoing a full colonoscopy. Contemp Clin Trials 2018;71:96-102. [Crossref] [PubMed]
- Inoue H, Kudo SE, Shiokawa A. Novel endoscopic imaging techniques toward in vivo observation of living cancer cells in the gastrointestinal tract. Clin Gastroenterol Hepatol 2005;3:S61-3. [Crossref] [PubMed]
- Tomizawa Y, Abdulla HM, Prasad GA, et al. Endocytoscopy in esophageal cancer. Gastrointest Endosc Clin N Am 2009;19:273-81. [Crossref] [PubMed]
- Kudo SE, Wakamura K, Ikehara N, et al. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy 2011;43:869-75. [Crossref] [PubMed]
- Kumagai Y, Kawada K, Yamazaki S, et al. Endocytoscopic observation of esophageal squamous cell carcinoma. Dig Endosc 2010;22:10-6. [Crossref] [PubMed]
- Fujishiro M, Kodashima S, Takubo K, et al. Detailed comparison between endocytoscopy and horizontal histology of an esophageal intraepithelial squamous cell carcinoma. Dis Esophagus 2008;21:181-5. [Crossref] [PubMed]
- Kodashima S, Fujishiro M, Takubo K, et al. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy 2006;38:1115-21. [Crossref] [PubMed]
- Pohl H, Koch M, Khalifa A, et al. Evaluation of endocytoscopy in the surveillance of patients with Barrett's esophagus. Endoscopy 2007;39:492-6. [Crossref] [PubMed]
- Eleftheriadis N, Inoue H, Ikeda H, et al. Endocytoscopic visualization of squamous cell islands within Barrett's epithelium. World J Gastrointest Endosc 2013;5:174-9. [Crossref] [PubMed]
- Tomizawa Y, Iyer PG, Wongkeesong LM, et al. Assessment of the diagnostic performance and interobserver variability of endocytoscopy in Barrett's esophagus: a pilot ex-vivo study. World J Gastroenterol 2013;19:8652-8. [Crossref] [PubMed]
- Inoue H, Sasajima K, Kaga M, et al. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy 2006;38:891-5. [Crossref] [PubMed]
- Kumagai Y, Kawada K, Yamazaki S, et al. Endocytoscopic observation for esophageal squamous cell carcinoma: can biopsy histology be omitted? Dis Esophagus 2009;22:505-12. [Crossref] [PubMed]
- Kumagai Y, Kawada K, Yamazaki S, et al. Current status and limitations of the newly developed endocytoscope GIF-Y0002 with reference to its diagnostic performance for common esophageal lesions. J Dig Dis 2012;13:393-400. [Crossref] [PubMed]
- Kaise M, Kimura R, Nomura K, et al. Accuracy and concordance of endocytoscopic atypia for the diagnosis of gastric cancer. Endoscopy 2014;46:827-32. [Crossref] [PubMed]
- Sato H, Inoue H, Ikeda H, et al. In vivo gastric mucosal histopathology using endocytoscopy. World J Gastroenterol 2015;21:5002-8. [Crossref] [PubMed]
- Chiu PW, Ng EK, To KF, et al. Recognition of goblet cells upon endocytoscopy indicates the presence of gastric intestinal metaplasia. Dig Endosc 2014;26:52-6. [Crossref] [PubMed]
- Sato H, Inoue H, Hayee B, et al. In vivo histopathology using endocytoscopy for non-neoplastic changes in the gastric mucosa: a prospective pilot study (with video). Gastrointest Endosc 2015;81:875-81. [Crossref] [PubMed]
- Kutsukawa M, Kudo SE, Ikehara N, et al. Efficiency of endocytoscopy in differentiating types of serrated polyps. Gastrointest Endosc 2014;79:648-56. [Crossref] [PubMed]
- Kimura S, Inoue H, Sato Y, et al. Ex vivo visualization of Helicobacter pylori using an endocytoscopic probe. Biomed Res 2006;27:255-7. [Crossref] [PubMed]
- Fasoli A, Pugliese V, Furnari M, et al. Signet ring cell carcinoma of the stomach: correlation between endocytoscopy and histology. Endoscopy 2009;41 Suppl 2:E65-6. [Crossref] [PubMed]
- Isomoto H, Matsushima K, Hayashi T, et al. Endocytoscopic findings of lymphomas of the stomach. BMC Gastroenterol 2013;13:174. [Crossref] [PubMed]
- Inoue H, Kazawa T, Sato Y, et al. In vivo observation of living cancer cells in the esophagus, stomach, and colon using catheter-type contact endoscope, "Endo-Cytoscopy system Gastrointest Endosc Clin N Am 2004;14:589-94. x-xi. [Crossref] [PubMed]
- Sako T, Kudo SE, Miyachi H, et al. A novel ability of endocytoscopy to diagnose histological grade of differentiation in T1 colorectal carcinomas. Endoscopy 2018;50:69-74. [PubMed]
Cite this article as: Abad MR, Shimamura Y, Fujiyoshi Y, Seewald S, Inoue H. Endocytoscopy: technology and clinical application in upper gastrointestinal tract. Transl Gastroenterol Hepatol 2020;5:28.


