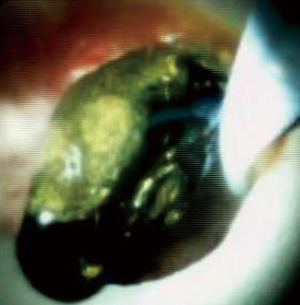
Assessing the utility, findings, and outcomes of percutaneous transhepatic cholangioscopy with SpyglassTM Direct visualization system: a case series
Introduction
Patients with altered luminal anatomy who require biliary drainage, sampling or other diagnostic and therapeutic interventions may not be suitable for the traditional endoscopic retrograde cholangiopancreatography (ERCP) (1,2). Methods to endoscopically access the remnant stomach, including enteroscopy-assisted ERCP or laparoscopy-assisted ERCP in gastric bypass patients (3), and/or endoscopic ultrasound guided gastro-gastric fistula creation (4), have been described with variable success rate in the literature. We hereby describe an alternative approach that allows access to the biliary tree with direct visualization and sampling of the bile duct via cholangioscopy; this novel approach additionally facilitates optically guided intraductal fragmentation and clearance of biliary calculi (1,5). This case series identifies five patients who successfully underwent percutaneous transhepatic cholangioscopy (PTCS) with SpyglassTM Direct visualization system (SpyglassTM DS Boston Scientific Corporation, Marlborough, Massachusetts). Since SpyglassTM scope requires at least a 12 French (Fr) percutaneous tract, the initial 8 Fr percutaneous transhepatic biliary drainage (PTBD) catheter was upsized to 12 Fr and the bile duct was explored endoscopically via a standard 12 Fr percutaneous vascular sheath once the tract matured. We present the following case series in accordance with the CARE-Guideline (6).
Patient case 1
A 59-year-old male with liver transplant with Roux-en-Y hepaticojejunostomy in 2008 and re-transplant in 2015 for chronic rejection presented with fever of 38.6 °C. He was found to have obstructive pattern liver enzymes; blood cultures during the admission were positive for Escherichia coli bacteremia, and diagnosis of cholangitis was made. Magnetic resonance cholangiopancreatography (MRCP) revealed anastomotic stricture at hepaticojejunostomy and choledocholithiasis at allograft bile duct. ERCP was not attempted due to the altered anatomy. Percutaneous transhepatic cholangiography (PTCS) revealed marked dilatation of the common bile duct (CBD) with large stones. PTCS was performed with the SpyglassTM introduced and advanced to the lower third of the main duct. The lower third of the main bile duct and middle third of the main bile duct contained two stones, the larger of which was 10 mm in diameter. EHL was performed successfully and hepaticojejunostomy anastomosis was visualized and crossed with SpyglassTM without difficulty. Using balloon sweep, the stones were swept from the bile duct into the anastomosis by interventional radiology (IR); post-procedure cholangioscopy demonstrated a patent anastomosis with resolution of large indwelling stones. Eleven weeks later, over the wire PTCS revealed no filling defect. However, a stenosis involving hepaticojejunostomy resulting in markedly slow flow of contrast to the bowel, which improved with 14 Fr placement, was identified. In the subsequent two months, a cholangiogram through the existing PTBD catheter demonstrated anastomotic stricture despite a patent anastomosis. Stricture cholangioplasty was performed with a new over the wire 14 Fr internal/external biliary drainage catheter placement. A cholangiogram, ten weeks later, did not reveal any filling defects as the contrast was observed freely draining into the bowel from the biliary tree; thus, the PTBD catheter was removed. Patient experienced recurrent cholangitis until hepaticojejunostomy stricture was corrected; thereafter, no further symptoms were present on follow-up.
Patient case 2
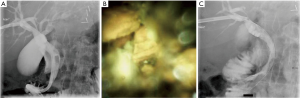
A 74-year-old male with Roux-en-Y gastric bypass presented with severe acute cholangitis and hyperbilirubinemia due to choledocholithiasis. Due to altered anatomy and urgent need for biliary decompression, the patient had a temporizing 8 Fr internal-external PTBD catheter placed. A month later, after upsizing the PTBD catheter to 12 Fr, the SpyglassTM was advanced percutaneously to the lower third of the main duct. The upper third of the CBD contained three stones as revealed in Figure 1A, which were successfully fragmented via electrohydraulic lithotripsy (EHL). Duct clearance was achieved by balloon sweep performed by IR per Figure 1B. A few weeks later, a cholangiogram performed through the existing drainage catheter demonstrated no filling defects within the CBD or the remaining biliary tree as seen in Figure 1C. Subsequently, the percutaneous biliary drain was removed.
Patient case 3
A 52-year-old male with hepatitis C and alcoholic liver cirrhosis status post orthotopic liver transplant (OLT) in 2006 presented with persistent jaundice, pruritus and weakness. Patient had been admitted with severe biliary stricture a year earlier and was being managed with PTBD; however, he failed to follow-up after the biliary drainage catheter accidentally dislodged. MRCP revealed choledochojejunostomy post OLT, mild intrahepatic biliary ductal dilatation, and 20 mm hypointense filling defects in the common hepatic duct just proximal to the anastomosis likely representing an intraductal calculus versus a biliary polyp per Figure 2. Given recurrent biliary obstruction, a PTBD catheter was placed to facilitate SDVS. The cholangioscope was inserted via a percutaneous 12 Fr vascular sheath into the distal CBD. The bile duct was explored endoscopically. Evidence of a previous surgical anastomosis was seen in the middle third of the main bile duct. Mild erythema at this site was considered to reveal granulation tissue and biopsies were obtained for confirmation. Biopsy revealed small bowel mucosa with patchy chronic inflammation without evidence of malignancy. EHL was performed for stone fragmentations. Follow up cholangiogram demonstrated contrast freely draining from biliary system into small bowel; thus, the biliary drainage catheter was removed. Patient responded well at twelve months follow-up post-PTCS with SDVS.
Patient case 4
A 78-year-old male with an eight-year history of adenocarcinoma of gallbladder status post chemo-radiation, laparoscopic cholecystectomy, segmental resection of liver, gallbladder and extrahepatic bile duct resection and Roux-en-Y hepaticojejunostomy presented with six-week history of abdominal pain and twenty-pound weight loss. Imaging studies including contrast enhanced computed tomography (CT) abdomen and pelvis, and MRCP revealed ill-defined 25 mm low-density lesion at the hilum with moderate upstream biliary ductal dilation. Attempts at ERCP failed to reach the Roux limb. Internal-external PTBD catheter was placed and percutaneous cholangioscopy evaluation with SDVS was performed. This showed abnormal mucosa with prominent mucosal vessels and erythema at the hepaticojejunostomy anastomosis indicating presence of stricture. Biopsies were sent for histology and fluorescence in situ hybridization (FISH) testing, which revealed fragments of markedly inflamed and reactive mucosa with marked crush artifact; however, no tumor was identified and FISH testing resulted negative. Once biliary stasis resolved; the drainage catheter was removed with no consequences. Patient showed no evidence of obstruction twelve months post-PTCS.
Patient case 5
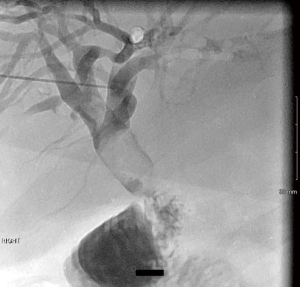
A 54-year-old female with factor II deficiency on warfarin, portal vein thrombosis, recurrent liver enzyme elevations, and Roux-en-Y gastric bypass presented with obstructive pattern liver enzymes. CT and MRCP revealed cholelithiasis and intrahepatic biliary ductal dilatation with CBD of up to 11 mm and a small obstructive CBD stone. PTCS demonstrated filling defect at the proximal CBD measuring about 5 mm by 9 mm, compatible with choledocholithiasis as seen in Figure 3. Internal-external PTBD was placed and subsequently the bile duct was explored with the SpyglassTM via PTCS. The main duct contained multiple stones. EHL fragmented the biliary stones and multiple sweeps of the CBD were performed to clear debris and stones. She did well on follow up with no further symptoms and was referred for cholecystectomy.
Table 1 summarizes SDVS utility, findings and outcomes associated with all five cases reported in this case study.

Full table
Discussion
In mixed restrictive-malabsorptive bariatric procedures (gastric bypass and biliopancreatic diversion), the anatomy reaching the biliary system is significantly altered. Nonetheless, ERCP is challenging yet possible after gastric bypass either as EUS directed transgastric ERCP (EDGE) or laparoscopic assisted enteroscopy; on the other hand, following biliopancreatic diversion, the presence of interposed intestinal segment renders ERCP impossible (3,4). Bariatric surgeries, in addition to obesity, pose significant risk of cholelithiasis and choledocholithiasis (7). In these high risk and challenging patients, PTBD catheter offers a valuable access for SpyglassTM technique (2,8). PTCS with SDVS is described in Table 2.

Full table
Post liver transplant biliary complications represent another indication for endoscopic interventions (9). Increase in incidence of liver transplants along with successfully prolonged survival post-transplant has subsequently increased prevalence of transplant cases. Appropriateness of specific endoscopic intervention varies by the type of biliary reconstruction following transplant. In choledocho-choledochostomy, ERCP may be performed; however, in duct-to-duct anastomosis and Roux-en-Y, hepaticojejunostomy or choledochojejunostomy percutaneous cholangioscopy is required (9,10,11).
Accurate diagnosis of biliary strictures is similarly enhanced with the use of SpyglassTM cholangioscopy as it provides improved visibility and biopsy capabilities (12). Moreover, SpyglassTM cholangioscopy is valuable for clearance of biliary ductal stones not effectively removed by traditional maneuvers (4).
Compared to ERCP, PTCS is invasive, time consuming and painful; nonetheless, it offers a less invasive alternative to open or laparoscopic surgeries, which are associated with greater complication risks (13). SDVS offers direct visualization, and ability to obtain targeted biopsies and EHL under direct visualization (12). Moreover, several advantages of SDVS over traditional PTCS include: four-way steerability allowing improved maneuverability of the Spyglass, availability of independent irrigation channels to maintain clear cholangioscopic field, and single endoscopist operation (14,15). On the other hand, some shortcomings of SDVS include requirement of tract maturation prior to use, and fiber optic image quality inferior to ERCP video images (14). While the overall stone removal success rate using SDVS exceeds 90%, overall complication rate approaches only 5%, commonly including adverse events such as liver laceration, intra-abdominal abscess, cholangitis, acute pancreatitis, hemobilia, bile duct perforation, disruption of the PTBD fistula and septic shock (5,8). These complications can further be minimized by allowing PTCS tracts to mature for at least 2 weeks and gradually dilating it prior to SDVS (13). Additional limitations of SDVS include contraindication in active cholangitis to minimize intrahepatic abscess, bacteremia and sepsis; differing levels of SDVS operator experience and expertise may significantly result in outcome variability; moreover, PTBD tube must be maintained in place throughout the treatment period (1,2,5).
In conclusion, PTCS can be beneficial for multiple diagnostic and therapeutic indications especially in patients with altered surgical anatomy. Some indications for PTCS include evaluation of biliary strictures (benign or malignant), biliary tract biopsy, lithotripsy and removal of biliary stones, and undiagnosed biliary stasis evaluation.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. No patient identifiers. Verbal informed consent obtained from patients.
References
- Nakai Y, Kogure H, Yamada A, et al. Endoscopic management of bile duct stones in patients with surgically altered anatomy. Dig Endosc 2018;30 Suppl 1:67-74. [Crossref] [PubMed]
- Du L, D'Souza P, Thiesen A, et al. Percutaneous transhepatic cholangioscopy for indeterminate biliary strictures using the SpyGlass system: a case series. Endoscopy 2015;47:1054-6. [Crossref] [PubMed]
- Abbas AM, Strong AT, Diehl DL, et al. Multicenter evaluation of the clinical utility of laparoscopy-assisted ERCP in patients with Roux-en-Y gastric bypass. Gastrointest Endosc 2018;87:1031-9. [Crossref] [PubMed]
- Kedia P, Kumta NA, Widmer J, et al. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: a novel technique. Endoscopy 2015;47:159-63. [Crossref] [PubMed]
- Laleman W, Verraes K, Van Steenbergen W, et al. Usefulness of the single-operator cholangioscopy system SpyGlass in biliary disease: a single-center prospective cohort study and aggregated review. Surg Endosc 2017;31:2223-32. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Li VK, Pulido N, Fajnwaks P, et al. Predictors of gallstone formation after bariatric surgery: a multivariate analysis of risk factors comparing gastric bypass, gastric banding, and sleeve gastrectomy. Surg Endosc 2009;23:1640-4. [Crossref] [PubMed]
- Franzini T, Cardarelli-Leite L, Figueira ERR, et al. SpyGlass percutaneous transhepatic cholangioscopy-guided lithotripsy of a large intrahepatic stone. Endoscopy 2017;49:E292-3. [Crossref] [PubMed]
- Hüsing-Kabar A, Heinzow HS, Schmidt HH, et al. Single-operator cholangioscopy for biliary complications in liver transplant recipients. World J Gastroenterol 2017;23:4064-71. [Crossref] [PubMed]
- Arain MA, Attam R, Freeman ML. Advances in endoscopic management of biliary tract complications after liver transplantation. Liver Transpl 2013;19:482-98. [Crossref] [PubMed]
- Girotra M, Soota K, Klair JS, et al. Endoscopic management of post-liver transplant biliary complications. World J Gastrointest Endosc 2015;7:446-59. [Crossref] [PubMed]
- Draganov P. The SpyGlass® Direct Visualization System for Cholangioscopy. Gastroenterol Hepatol (N Y) 2008;4:469-70. [PubMed]
- Chen C, Huang M, Yang J, et al. Reappraisal of percutaneous transhepatic cholangioscopic lithotomy for primary hepatolithiasis. Surg Endosc 2005;19:505-9. [Crossref] [PubMed]
- Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization,and biopsy. Gastrointest Endosc 2007;65:303-11. [Crossref] [PubMed]
- Yasuda I, Itoi T. Recent advances in endoscopic management of difficult bile duct stones. Dig Endosc 2013;25:376-85. [Crossref] [PubMed]
Cite this article as: Tripathi N, Mardini H, Koirala N, Raissi D, Emhmed Ali SM, Frandah WM. Assessing the utility, findings, and outcomes of percutaneous transhepatic cholangioscopy with SpyglassTM Direct visualization system: a case series. Transl Gastroenterol Hepatol 2020;5:12.