
Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer
Introduction
Gastrectomy is the most effective treatment for gastric cancer (GC) without distant metastasis (1). However, it is associated with the occurrence of postoperative complications, cancer recurrence, and death from other diseases. In addition, the post-surgical incidence of complications and survival rate among patients with similar cancer stage and demographics is heterogeneous. Identifying prognostic factors that allow a more accurate stratification of the preoperative risk remains an interest of GC experts.
To date, various factors such as advanced age, comorbidities, and molecular features have been reported to be associated with unfavorable outcomes following gastrectomy in patients with GC (2,3). However, most of those factors are difficult to manipulate preoperatively. Identifying modifiable factors is therefore necessary to overcome morbidity and cancer death in patients with GC.
Malnutrition is a critical problem, not only in patients with advanced GC, who can suffer from gastric outlet obstruction and bleeding, but also in patients with early GC, as their stomach presents reduced digestive capacity, causing food intake to decrease after gastrectomy (4,5). In addition, neoadjuvant chemotherapy and chemoradiation therapy, which often worsen a patient’s nutritional status, have become a standard in Western countries. Preoperative malnutrition therefore has significant room for improvement and several studies have investigated parameters to identify patients at nutritional risk. However, no universally accepted marker that is convenient for clinical use in patients with GC has been defined.
In patients with cancer, body mass composition and associated metabolic dysfunction have become an important preoperative consideration to predict postoperative outcomes. Since patients with GC often have unique metabolic problems, GC experts should understand body composition analysis. In this study, we conducted a literature review and introduce a method to evaluate body composition and the relationship between skeletal muscle mass and GC.
Challenges in defining body mass composition
The body mass index (BMI) has been used for a long time to diagnose malnutrition. Low BMI has been commonly recognized as a marker for poor nutrition status, whereas high BMI is associated with cardiovascular and kidney diseases and diabetes, which is also an important risk factor. However, previous studies investigating the relationship between BMI and clinical outcomes in patients with GC have provided inconsistent results (5-10).
Weight gain and loss are not reliable indicators of body composition changes, due to fluid collection such as ascites or body edema. Males are likely to store body fat in the visceral area while females store it mainly subcutaneously. In addition, people tend to lose muscle mass and gain fat as they get older. Therefore, patients with similar BMIs can have different nutritional status.
Body composition, which is estimated through various simple techniques and precisely reflects a patient’s metabolic profile, has recently attracted considerable attention. The clinical role of skeletal muscle mass is being increasingly recognized in patients with various cancers, and the amount of skeletal muscle mass is significantly associated with the post-surgical risk of complications, hospital stay, healthcare costs, and survival (11-18). Understanding the body composition analysis of a patient with GC and the relationship between GC and skeletal muscle mass can help address the potential metabolic problems encountered during the perioperative period.
Sarcopenia
Life expectancy has increased worldwide and aging is known to be associated with progressive loss of muscle mass (19,20). Muscle loss starts at 50 years of age and approximately 50% of the fibers is lost by 80 years of age (21). The term ‘sarcopenia’ (Greek ‘sarx’ or flesh + ‘penia’ or loss) was first proposed by Rosenberg in 1989, to designate the loss of skeletal muscle mass with aging (22). Sarcopenia can be classified into primary, which is caused by aging, and secondary, which is caused by immobility or diseases such as cancer (23). Moreover, sarcopenia can be also caused by both malnutrition, low levels of physical activity, and various diseases (24,25). Since sarcopenia may be caused by insufficient energy and protein intake, its prevalence in patients with GC with decreased food intake can be higher than in other cancer patients (26,27).
Muscle mass in patients with GC can be measured using two common approaches: bioelectrical impedance analysis (BIA) and computed tomography (CT). Dual-energy X-ray absorptiometry, another common method to measure muscle mass, is rarely used in the surgical field. Although BIA avoids exposing the patient to high radiation doses, it is influenced by the patient's fluid status. Although CT exposes the patient to a high radiation dose and requires expensive equipment and image analysis software, it is readily applicable to patients with GC during CT scan for staging and follow-up. Therefore, its clinical use is very convenient for clinical use for surgical candidates, as it requires no extra cost. Additionally, CT scans constitute a precise and gold standard tool to detect sarcopenia (28).
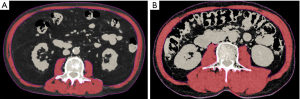
The authors have often adopted CT to identify sarcopenia (29-31). Skeletal muscle, visceral fat, and other tissues can be distinguished using Hounsfield units (HUs), with skeletal muscle’s cross-sectional area falling within the range from −29 to 150 HU. Representative images are shown in Figure 1, which includes the psoas, erector spinae, quadratus, lumborum, transversus abdominis, external and internal obliques, and rectus abdominis muscles. This landmark is known to correlate with whole-body muscle mass (32).
To calculate the skeletal muscle mass index (SMI), skeletal muscle mass assessed by BIA or CT scan is divided by the square of the patient’s height. Sex-specific SMI cut-offs are used to diagnose sarcopenia. Some researchers have used other methods and criteria, such as muscle quality, to report significant clinical impact of skeletal muscle in patients with GC (33-35).
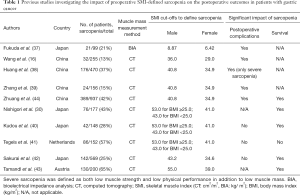
Previous studies (16,30,36-44) examining the relationship between SMI and clinical outcomes in patients with GC undergoing surgery are summarized in Table 1. Many studies from East Asia have reported an association between preoperative sarcopenia and a higher rate of postoperative complications and a poorer prognosis (16,30,36-44). In those studies, the prevalence of sarcopenia ranged from 13% to 65%, which reflects different countries and ethnicities as well as the unavailability of universally accepted cut-off values to define sarcopenia.
Full table
As shown in Table 1, the cut-off values reported by Zhuang et al. were commonly used in Chinese populations (44). The authors performed a study to determine the optimal sarcopenia cut-off values and to predict the long-term survival of Japanese patients with GC (30). Five definitions of CT-based SMI, previously reported to be associated with prognosis in cancer patients were used to define sarcopenia (16,40,42,44,45). The BMI-incorporated cut-off values reported by Martin et al. were associated with survival rate, both in univariate and multivariate analyses (40,46). Although Tegels et al. reported sarcopenia based on Martin et al.’s definition, which was not associated with postoperative outcomes (41), the prognostic significance of sarcopenia based on the cut-offs reported by Martin et al. has been validated in a number of malignancies (40,46-51). The authors believe that this BMI-incorporated definition can be used to determine sarcopenia in various countries and ethnicities.
According to a previous systematic review, preoperative sarcopenia diagnosed according to various definitions has significant impact on the short- and long-term postoperative outcome of GC patients (52). However, the best sarcopenia cut-offs should be determined to realize preoperative risk stratification and counter-measures, as well as to promote shared decision-making.
Several previous studies have identified sarcopenia based on muscle mass alone. However, the European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group for Sarcopenia (AWGS), recommend the simultaneous evaluation of muscle strength or physical function, to diagnose sarcopenia (28,53). Muscle strength and physical function are commonly measured by grip strength and walking speed, respectively. In the previous studies shown in Table 1, Fukuda et al., Wang et al., and Huang et al. defined preoperative sarcopenia based on the EWGSOP or AWGS algorithms and demonstrated that it is a risk factor for postoperative outcomes (16,37,38). Future prospective studies investigating the impact of sarcopenia on clinical outcomes of patients with GC should evaluate those parameters simultaneously with skeletal muscle mass.
Although the mechanism linking preoperative sarcopenia to worse postoperative outcomes remains unclear, skeletal muscle has been reported to play a role as an endocrine organ, producing cytokines and peptides that affect the immune system (54,55). Sarcopenic populations have been shown to have impaired cellular immune function and increased inflammatory activity (56). Moreover, in patients with GC, higher neutrophil/lymphocyte and platelet/lymphocyte ratios have been shown to be associated with preoperative sarcopenia (57). An association between sarcopenia and chemotherapy toxicity and termination has also been reported (58-61). In patients with GC receiving chemotherapy for metastatic diseases, low SMI was an independent predictor of poor outcome (36,62). In addition, skeletal muscle is known to constitute a source of amino acids in times of stress (63). Taken together, these findings suggest that sarcopenic patients with GC who are unable to react appropriately to the stress of gastrectomy and chemotherapy have unfavorable postoperative outcomes.
Sarcopenic obesity
The worldwide population is aging and the incidence of obesity rises rapidly (64). The association between visceral fat and metabolic syndrome and other serious diseases is well known (65-68). Moreover, visceral obesity has been reported as associated with high complication rates in patients with GC undergoing surgery (31,69,70).
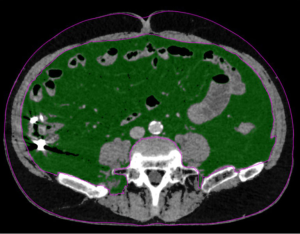
Sarcopenic obesity combines the health risks of obesity and sarcopenia, and is considered a worst-case scenario (71,72). Although the definition of obesity remains unclear, BMI and visceral fat mass are commonly used to define overweight and obesity (30,31,73,74). In Asian populations, BMI ≥25 or 30 kg/cm2 and visceral fat area ≥100 cm2 have been used as a threshold (30,31,75-79). Visceral fat mass can be measured by assessing the cross-sectional area at the level of the umbilicus on CT scan (32,80,81), where fat falls within the range of −190 to −30 HU (32). Figure 2 shows a representative CT image at the level of the umbilicus. In previous studies, sarcopenic obesity in patients with GC undergoing surgery was strongly associated with poor short- and long-term outcomes (30,31,73,74).
The mechanism linking sarcopenic obesity and poor outcomes also remains unclear. The inflammatory cytokines produced by adipose tissue, especially visceral fat, accelerate muscle catabolism, thereby contributing for the vicious cycle that initiates and sustains sarcopenic obesity (82). This, in turn, is linked with insulin resistance and dysmetabolism (83,84). In sarcopenic obesity patients, the response to the stress of surgery and chemotherapy may be further impaired, resulting in increased complications and poor prognosis.
As shown Figure 1, patients with obesity and muscle loss could not be distinguished by appearance, unlike cachexic or underweight ones. Moreover, assessing skeletal muscle mass in patients with GC who are not thin, is very important to stratify morbidity and mortality outcomes.
Interventions for sarcopenia
In sarcopenic patients with GC, preoperative interventions to improve body composition may lead to favorable outcomes. Fukuda et al. reported that sarcopenic patients with GC consumed less energy and protein preoperatively (37). In addition to resistance training, adequate intake of energy and protein is crucial to manage sarcopenia (85,86). Protein supplementation during resistance training, in particular the intake of essential amino acids such as leucine, was reported to increase protein synthesis and skeletal muscle mass (87-90). Although time to surgery is limited in preoperative patients with GC, exercise training was found to improve exercise capacity even during short preoperative periods (91-93). Yamamoto et al. reported that 3 weeks of preoperative exercise and nutritional therapy reduced sarcopenia and improved the postoperative outcomes of patients with GC and sarcopenia (94). They used HMB supplementation during resistance training, which was reported to effectively increase the lean body mass and to decrease muscle damage (95,96). Regarding interventions for sarcopenic obesity, energy intake restriction on fat-mass loss alone should not be performed, as it was reported to further decrease skeletal muscle mass (97,98). Cho et al. reported that preoperative exercise without diet control reduced visceral fat mass and postoperative complications following gastrectomy, in patients with GC and metabolic syndrome (99). Since few reports have looked into the effects of perioperative nutrition therapy during resistance training, in patients with GC and sarcopenia or sarcopenic obesity, further studies are necessary.
Conclusions
Identifying sarcopenic patients has several potential benefits in terms of GC treatment management. Currently, body composition can be easily evaluated using CT or BIA. A previous study reported that over half of patients with GC undergoing gastrectomy lost 5% or more of lean body mass one month after surgery (100). Loss of skeletal muscle mass is known to be associated with functional limitations, physical disability and decreased quality of life (101). In addition, loss of skeletal muscle mass after gastrectomy has been shown to impair compliance with adjuvant chemotherapy, resulting in poor prognosis (102-104). Medical workers involved in GC treatment should understand the significant role of skeletal muscle mass and perform constant body composition assessment before and after surgery and chemotherapy. In addition, patients with GC and sarcopenia should be provided appropriate energy and protein intake during resistance training, to improve short- and long-term outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- Asplund J, Kauppila JH, Mattsson F, et al. Survival Trends in Gastric Adenocarcinoma: A Population-Based Study in Sweden. Ann Surg Oncol 2018;25:2693-702. [Crossref] [PubMed]
- Seo JY, Jin EH, Jo HJ, et al. Clinicopathologic and molecular features associated with patient age in gastric cancer. World J Gastroenterol 2015;21:6905-13. [Crossref] [PubMed]
- Fujiya K, Kawamura T, Omae K, et al. Impact of Malnutrition After Gastrectomy for Gastric Cancer on Long-Term Survival. Ann Surg Oncol 2018;25:974-83. [Crossref] [PubMed]
- Kubo H, Komatsu S, Ichikawa D, et al. Impact of Body Weight Loss on Recurrence After Curative Gastrectomy for Gastric Cancer. Anticancer Res 2016;36:807-13. [PubMed]
- Lee JH, Park B, Joo J, et al. Body mass index and mortality in patients with gastric cancer: a large cohort study. Gastric Cancer 2018;21:913-24. [Crossref] [PubMed]
- Feng F, Zheng G, Guo X, et al. Impact of body mass index on surgical outcomes of gastric cancer. BMC Cancer 2018;18:151. [Crossref] [PubMed]
- Kulig J, Sierzega M, Kolodziejczyk P, et al. Implications of overweight in gastric cancer: A multicenter study in a Western patient population. Eur J Surg Oncol 2010;36:969-76. [Crossref] [PubMed]
- Tokunaga M, Hiki N, Fukunaga T, et al. Better 5-Year Survival Rate Following Curative Gastrectomy in Overweight Patients. Ann Surg Oncol 2009;16:3245-51. [Crossref] [PubMed]
- Bickenbach KA, Denton B, Gonen M, et al. Impact of Obesity on Perioperative Complications and Long-term Survival of Patients with Gastric Cancer. Ann Surg Oncol 2013;20:780-7. [Crossref] [PubMed]
- Norman K, Otten L. Financial impact of sarcopenia or low muscle mass - A short review. Clin Nutr 2019;38:1489-95. [Crossref] [PubMed]
- Sheetz KH, Waits SA, Terjimanian MN, et al. Cost of Major Surgery in the Sarcopenic Patient. J Am Coll Surg 2013;217:813-8. [Crossref] [PubMed]
- Ongaro E, Buoro V, Cinausero M, et al. Sarcopenia in gastric cancer: when the loss costs too much. Gastric Cancer 2017;20:563-72. [Crossref] [PubMed]
- Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931-6. [Crossref] [PubMed]
- Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann Surg Oncol 2015;22:2663-8. [Crossref] [PubMed]
- Wang SL, Zhuang CL, Huang DD, et al. Sarcopenia Adversely Impacts Postoperative Clinical Outcomes Following Gastrectomy in Patients with Gastric Cancer: A Prospective Study. Ann Surg Oncol 2016;23:556-64. [Crossref] [PubMed]
- Joglekar S, Asghar A, Mott SL, et al. Sarcopenia Is an Independent Predictor of Complications Following Pancreatectomy for Adenocarcinoma. J Surg Oncol 2015;111:771-5. [Crossref] [PubMed]
- Harimoto N, Shirabe K, Yamashita YI, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 2013;100:1523-30. [Crossref] [PubMed]
- Christensen K, Doblhammer G, Rau R, et al. Ageing populations: the challenges ahead. Lancet 2009;374:1196-208. [Crossref] [PubMed]
- Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol (1985) 2003;95:1717-27. [PubMed]
- Faulkner JA, Larkin LM, Claflin DR, et al. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol 2007;34:1091-6. [Crossref] [PubMed]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990S-991S. [Crossref] [PubMed]
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 2014;11:177-80. [PubMed]
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755-63. [Crossref] [PubMed]
- Muscaritoli M, Anker SD, Argiles J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr 2010;29:154-9. [Crossref] [PubMed]
- Scott D, Blizzard L, Fell J, et al. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: the Tasmanian Older Adult Cohort Study. J Am Geriatr Soc 2010;58:2129-34. [Crossref] [PubMed]
- Valenzuela RE, Ponce JA, Morales-Figueroa GG, et al. Insufficient amounts and inadequate distribution of dietary protein intake in apparently healthy older adults in a developing country: implications for dietary strategies to prevent sarcopenia. Clin Interv Aging 2013;8:1143-8. [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [Crossref] [PubMed]
- Nishigori T, Okabe H, Tanaka E, et al. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol 2016;113:678-84. [Crossref] [PubMed]
- Nishigori T, Tsunoda S, Obama K, et al. Optimal Cutoff Values of Skeletal Muscle Index to Define Sarcopenia for Prediction of Survival in Patients with Advanced Gastric Cancer. Ann Surg Oncol 2018;25:3596-603. [Crossref] [PubMed]
- Nishigori T, Tsunoda S, Okabe H, et al. Impact of Sarcopenic Obesity on Surgical Site Infection after Laparoscopic Total Gastrectomy. Ann Surg Oncol 2016;23:524-31. [Crossref] [PubMed]
- Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997-1006. [Crossref] [PubMed]
- Kuwada K, Kuroda S, Kikuchi S, et al. Sarcopenia and Comorbidity in Gastric Cancer Surgery as a Useful Combined Factor to Predict Eventual Death from Other Causes. Ann Surg Oncol 2018;25:1160-6. [Crossref] [PubMed]
- Kawamura T, Makuuchi R, Tokunaga M, et al. Long-Term Outcomes of Gastric Cancer Patients with Preoperative Sarcopenia. Ann Surg Oncol 2018;25:1625-32. [Crossref] [PubMed]
- Lu J, Zheng ZF, Li P, et al. A Novel Preoperative Skeletal Muscle Measure as a Predictor of Postoperative Complications, Long-Term Survival and Tumor Recurrence for Patients with Gastric Cancer After Radical Gastrectomy. Ann Surg Oncol 2018;25:439-48. [Crossref] [PubMed]
- Lee JS, Kim YS, Kim EY, et al. Prognostic significance of CT-determined sarcopenia in patients with advanced gastric cancer. PLoS One 2018;13:e0202700. [Crossref] [PubMed]
- Fukuda Y, Yamamoto K, Hirao M, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer 2016;19:986-93. [Crossref] [PubMed]
- Huang DD, Chen XX, Chen X, et al. Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: a prospective study. J Cancer Res Clin Oncol 2016;142:2347-56. [Crossref] [PubMed]
- Zhang Y, Wang JP, Wang XL, et al. Computed tomography-quantified body composition predicts short-term outcomes after gastrectomy in gastric cancer. Curr Oncol 2018;25:e411-22. [Crossref] [PubMed]
- Kudou K, Saeki H, Nakashima Y, et al. Prognostic Significance of Sarcopenia in Patients with Esophagogastric Junction Cancer or Upper Gastric Cancer. Ann Surg Oncol 2017;24:1804-10. [Crossref] [PubMed]
- Tegels JJ, van Vugt JL, Reisinger KW, et al. Sarcopenia Is Highly Prevalent in Patients Undergoing Surgery for Gastric Cancer But Not Associated With Worse Outcomes. J Surg Oncol 2015;112:403-7. [Crossref] [PubMed]
- Sakurai K, Kubo N, Tamura T, et al. Adverse Effects of Low Preoperative Skeletal Muscle Mass in Patients Undergoing Gastrectomy for Gastric Cancer. Ann Surg Oncol 2017;24:2712-9. [Crossref] [PubMed]
- Tamandl D, Paireder M, Asari R, et al. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol 2016;26:1359-67. [Crossref] [PubMed]
- Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore) 2016;95:e3164. [Crossref] [PubMed]
- Prado CM, Liefers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629-35. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Sharma P, Zargar-Shoshtari K, Caracciolo JT, et al. Sarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Urol Oncol 2015;33:339.e17-23. [Crossref] [PubMed]
- Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861-70. [Crossref] [PubMed]
- Fukushima H, Yokoyama M, Nakanishi Y, et al. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One 2015;10:e0115895. [Crossref] [PubMed]
- Fukushima H, Nakanishi Y, Kataoka M, et al. Prognostic Significance of Sarcopenia in Patients with Metastatic Renal Cell Carcinoma. J Urol 2016;195:26-32. [Crossref] [PubMed]
- Begini P, Gigante E, Antonelli G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol 2017;16:107-14. [Crossref]
- Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2019;22:10-22. [Crossref] [PubMed]
- Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101. [Crossref] [PubMed]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012;8:457-65. [Crossref] [PubMed]
- Pedersen BK, Bruunsgaard H. Possible beneficial role of exercise in modulating low-grade inflammation in the elderly. Scand J Med Sci Sports 2003;13:56-62. [Crossref] [PubMed]
- Bruunsgaard H, Pedersen BK. Special feature for the Olympics: effects of exercise on the immune system: effects of exercise on the immune system in the elderly population. Immunol Cell Biol 2000;78:523-31. [Crossref] [PubMed]
- Lin J, Zhang W, Huang Y, et al. Sarcopenia is associated with the neutrophil/lymphocyte and platelet/lymphocyte ratios in operable gastric cancer patients: a prospective study. Cancer Manag Res 2018;10:4935-44. [Crossref] [PubMed]
- Tan BH, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol 2015;41:333-8. [Crossref] [PubMed]
- Antoun S, Borget I, Lanoy E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care 2013;7:383-9. [Crossref] [PubMed]
- Prado CM, Baracos VE, McCargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 2007;13:3264-8. [Crossref] [PubMed]
- Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a Determinant of Chemotherapy Toxicity and Time to Tumor Progression in Metastatic Breast Cancer Patients Receiving Capecitabine Treatment. Clin Cancer Res 2009;15:2920-6. [Crossref] [PubMed]
- Hayashi N, Ando Y, Gyawali B, et al. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol Rep 2016;35:1727-31. [Crossref] [PubMed]
- Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. Curr Opin Clin Nutr Metab Care 2005;8:61-5. [Crossref] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377-96. [Crossref] [PubMed]
- Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010;33:920-2. [Crossref] [PubMed]
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881-7. [Crossref] [PubMed]
- Tsukada K, Miyazaki T, Kato H, et al. Body fat accumulation and postoperative complications after abdominal surgery. Am Surg 2004;70:347-51. [PubMed]
- Harada K, Baba Y, Ishimoto T, et al. Low Visceral Fat Content is Associated with Poor Prognosis in a Database of 507 Upper Gastrointestinal Cancers. Ann Surg Oncol 2015;22:3946-53. [Crossref] [PubMed]
- Kunisaki C, Makino H, Takagawa R, et al. Predictive factors for surgical complications of laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Endosc 2009;23:2085-93. [Crossref] [PubMed]
- Yoshikawa K, Shimada M, Kurita N, et al. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy-assisted gastrectomy for gastric cancer. Surg Endosc 2011;25:3825-30. [Crossref] [PubMed]
- Chung JY, Kang HT, Lee DC, et al. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch Gerontol Geriatr 2013;56:270-8. [Crossref] [PubMed]
- Lim S, Kim JH, Yoon JW, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010;33:1652-4. [Crossref] [PubMed]
- Zhang WT, Lin J, Chen WS, et al. Sarcopenic Obesity Is Associated with Severe Postoperative Complications in Gastric Cancer Patients Undergoing Gastrectomy: a Prospective Study. J Gastrointest Surg 2018;22:1861-9. [Crossref] [PubMed]
- Lou N, Chi CH, Chen XD, et al. Sarcopenia in overweight and obese patients is a predictive factor for postoperative complication in gastric cancer: A prospective study. Eur J Surg Oncol 2017;43:188-95. [Crossref] [PubMed]
- Lu CW, Yang KC, Chang HH, et al. Sarcopenic obesity is closely associated with metabolic syndrome. Obes Res Clin Pract 2013;7:e301-7. [Crossref] [PubMed]
- Lim KI, Yang SJ, Kim TN, et al. The association between the ratio of visceral fat to thigh muscle area and metabolic syndrome: the Korean Sarcopenic Obesity Study (KSOS). Clin Endocrinol (Oxf) 2010;73:588-94. [Crossref] [PubMed]
- Baek SJ, Nam GE, Han KD, et al. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008-2010 Korea National Health and Nutrition Examination Survey. J Endocrinol Invest 2014;37:247-60. [Crossref] [PubMed]
- Expert Consultation WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63. [Crossref] [PubMed]
- Kanazawa M, Yoshiike N, Osaka T, et al. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet 2005;94:1-12. [PubMed]
- Tokunaga K, Matsuzawa Y, Ishikawa K, et al. A novel technique for the determination of body fat by computed tomography. Int J Obes 1983;7:437-45. [PubMed]
- Kvist H, Chowdhury B, Sjostrom L, et al. Adipose tissue volume determination in males by computed tomography and 40K. Int J Obes 1988;12:249-66. [PubMed]
- Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985) 2007;102:919-25. [PubMed]
- Doyle SL, Donohoe CL, Lysaght J, et al. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc 2012;71:181-9. [Crossref] [PubMed]
- Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One 2010;5:e10805. [Crossref] [PubMed]
- Calvani R, Miccheli A, Landi F, et al. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J Frailty Aging 2013;2:38-53. [PubMed]
- Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150-5. [Crossref] [PubMed]
- Cermak NM, Res PT, de Groot LC, et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454-64. [Crossref] [PubMed]
- Kim HK, Suzuki T, Saito K, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc 2012;60:16-23. [Crossref] [PubMed]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291:E381-7. [Crossref] [PubMed]
- Volpi E, Kobayashi H, Sheffield-Moore M, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250-8. [Crossref] [PubMed]
- Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery 2011;150:505-14. [Crossref] [PubMed]
- Hoogeboom TJ, Dronkers JJ, van den Ende CH, et al. Preoperative therapeutic exercise in frail elderly scheduled for total hip replacement: a randomized pilot trial. Clin Rehabil 2010;24:901-10. [Crossref] [PubMed]
- Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 2013;27:1072-82. [Crossref] [PubMed]
- Yamamoto K, Nagatsuma Y, Fukuda Y, et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 2017;20:913-8. [Crossref] [PubMed]
- Panton LB, Rathmacher JA, Baier S, et al. Nutritional supplementation of the leucine metabolite beta-hydroxy-beta-methylbutyrate (hmb) during resistance training. Nutrition 2000;16:734-9. [Crossref] [PubMed]
- Nissen S, Sharp R, Ray M, et al. Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol (1985) 1996;81:2095-104. [PubMed]
- Chomentowski P, Dube JJ, Amati F, et al. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci 2009;64:575-80. [Crossref] [PubMed]
- Mason C, Xiao L, Imayama I, et al. Influence of diet, exercise, and serum vitamin d on sarcopenia in postmenopausal women. Med Sci Sports Exerc 2013;45:607-14. [Crossref] [PubMed]
- Cho H, Yoshikawa T, Oba MS, et al. Matched pair analysis to examine the effects of a planned preoperative exercise program in early gastric cancer patients with metabolic syndrome to reduce operative risk: the Adjuvant Exercise for General Elective Surgery (AEGES) study group. Ann Surg Oncol 2014;21:2044-50. [Crossref] [PubMed]
- Aoyama T, Sato T, Segami K, et al. Risk Factors for the Loss of Lean Body Mass After Gastrectomy for Gastric Cancer. Ann Surg Oncol 2016;23:1963-70. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Kugimiya N, Harada E, Oka K, et al. Loss of skeletal muscle mass after curative gastrectomy is a poor prognostic factor. Oncol Lett 2018;16:1341-7. [PubMed]
- Aoyama T, Kawabe T, Fujikawa H, et al. Loss of Lean Body Mass as an Independent Risk Factor for Continuation of S-1 Adjuvant Chemotherapy for Gastric Cancer. Ann Surg Oncol 2015;22:2560-6. [Crossref] [PubMed]
- Choi MH, Kim KA, Hwang SS, et al. CT-quantified muscle and fat change in patients after surgery or endoscopic resection for early gastric cancer and its impact on long-term outcomes. Medicine (Baltimore) 2018;97:e13878. [Crossref] [PubMed]
Cite this article as: Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol 2020;5:22.


