
Non-invasive assessment for alpha-1 antitrypsin deficiency-associated liver disease: new insights on steatosis and fibrosis in Pi*ZZ carriers
One in every 5,000 individuals is expected to be affected by the hereditary alpha-1 antitrypsin deficiency (AATD) predisposing to liver cirrhosis, hepatocellular carcinoma and chronic obstructive pulmonary disease (COPD). The gene encodes a 52 kDa protease inhibitor, a member of the serine protease inhibitor (SERPIN) superfamily of proteins synthesized in the liver. Despite its name, the main function of alpha-1 antitrypsin (AAT) is to inhibit neutrophil elastase (rather than trypsin) in the pulmonary tissue, thereby providing greater than 90% of the defense against the elastolytic burden in the lower airways (1). There are approximately 120 different alleles described for the normal Pi*MM (Pi for protease inhibitor) allele within the SERPINA1 gene on the long arm of chromosome 14 (14q31–32.3). The most common pathogenic mutation (>95% of clinically recognized cases) involves a substitution of lysine for glutamic acid at position 342 known as the Z allele, causing a deficiency of AAT characterized by serum levels below 80 mg/dL or <20 µM (reference range, 80–220 mg/dL or 20–53 µM) (2). This loss-of-function phenotype involves an imbalance of proteases and antiproteases in favor of the neutrophil elastase causing enhanced chemotactic activity and an accelerated breakdown of elastic tissue (particularly in the lung) and, ultimately, pulmonary emphysema (Figure 1). In hepatocytes, more specifically in their endoplasmic reticulum (ER), the Pi*ZZ mutation causes widening of the molecule’s β-sheet A, leading to polymerization of AAT by linking it irreversibly with the reactive loop of another AAT molecule and causing retention of the protein within the cell (i.e. , toxic-gain-of-function phenotype) (3). The retention of AAT inflicts chronic damage on the hepatocyte by inducing apoptosis, mitochondrial damage, inflammation and autophagy provoking repetitive regenerative stimuli on adjacent non-affected hepatocytes (Figure 1). This inflammation and proliferation stimulus triggers increased mitotic activity predisposing the tissue to fibrosis, cirrhosis and hepatocellular carcinoma (4). Clinically, the Pi*ZZ mutation predisposes to early onset (i.e., fourth and fifth decade) panacinar emphysema predominantly in the lung bases (vs. apical in usual smoking associated COPD). AATD associated liver disease includes hepatitis, cirrhosis, hepatoma and hepatocellular carcinoma and is linked to mutations causing intrahepatocyte AAT polymerization (5). The diagnostic approach in clinical practice comprises low serum AAT concentrations, AAT phenotyping using isoelectric focusing and genotyping (via PCR) (6). Liver biopsy reveals characteristic periodic acid Schiff- diastase (PAS-D) resistant globules within hepatocytes in AATD livers (7).
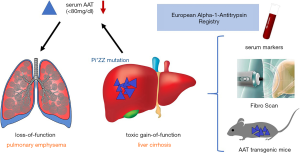
The peak incidence of liver and lung disease occur at different ages in Pi*ZZ patients (middle age in lung disease vs. neonatal and 6th life decade in liver disease) (8). The neonatal period is the first of two important peak incidence age groups for an AATD associated liver phenotype, typically presenting as a “neonatal hepatitis syndrome” that includes cholestatic jaundice, pruritus, poor feeding, poor weight gain, hepatomegaly, and splenomegaly (9,10). While there is extensive knowledge on AATD related lung disease in the literature, the data on AATD related liver disease is limited. The largest population-based screening study in Sweden has evaluated over 200,000 newborns, among which 127 Pi*ZZ carriers were identified. Twelve percent of those carriers developed obstructive jaundice and other liver dysfunctions in a 6-month follow-up. Twenty-five percent of these patients died in the first decade of life and 2% developed cirrhosis later in childhood (9). Follow-up of 70% of the Swedish Pi*ZZ carriers at the age of 30 interestingly revealed a low 3% to 5% rate of elevated liver transaminases without any clinically evident liver disease (11). This data supports the idea of a primary peak incidence of AATD associated liver disease and mortality in the neonatal period. The second peak of incidence was found in a study with 94 deceased Pi*ZZ AATD patients from Sweden, in which 35 demonstrated cirrhosis (mean age at death of 65.5±10.5 (SD) vs. 53.6±12.8 years for the 59 non-cirrhotic patients) in postmortem analysis. Fourteen of the 35 cirrhotic patients had hepatocellular carcinoma (12). After respiratory failure (45–72% of deaths), liver cirrhosis is the 2nd most common cause of death in patients with AATD (10-13% of deaths). Particularly among nonsmokers in whom the rate of lung disease is significantly lower, the prevalence of liver diseases rises with advancing age (13,14). The shortage of knowledge and the absence of clinical guidelines for AATD related liver disease together with its high morbidity and mortality emphasize the demand for prospective multicenter studies to establish clinical recommendations for the assessment and management of patients with AATD related liver disease.
With this aim in mind, the group of Strnad has conducted the largest prospective cohort study on AATD related liver disease by forming a European consortium (15). Using non-invasive diagnostic measures (common serological tests, scoring systems, vibration-controlled transient elastography (TE, FibroScan) and elastography-based controlled attenuation parameter (CAP) the hepatologic burden (liver fibrosis, steatosis) was assessed in an impressive number of 554 adult Pi*ZZ patients of European descent that were matched to their healthy, genetically unrelated spouses. Additionally, the group has performed histological and mechanistic analyses of transgenic mice overexpressing the Pi*Z allele (Figure 1).
In this large cohort, Pi*ZZ carriers have shown elevated serum liver enzymes and an increase in serum markers of liver fibrosis and steatosis, as compared to the healthy controls (15). No correlation was found between lung function and liver fibrosis, which confirms the current model of two different pathogenic mechanisms causally independent from each other (16). Male patients over the age of 50 with higher levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) or γ-glutamyl transferase (GGT) and low platelet levels were found to be the main risk group for liver fibrosis. A higher rate of steatosis was present in the Pi*ZZ group (38% vs. 31% in controls), as measured by CAP. Indicators for hepatic lipid secretion were reduced in the Pi*ZZ group, which was also found in the mouse model via histological presence of steatosis and down-regulation of genes encoding for lipid secreting compounds (15).
The remarkable effort to capture standardized data sets from Pi*ZZ patients all over Europe contributes to understanding liver disease manifestation and progression in AATD in order to establish guidelines and differentiate high risk from low risk patients. Also, the serum biomarkers used, both the commercially available Hepascore as well as the non-patented Aspartate Aminotransferase to Platelet Ratio Index (APRI) score, captivate with their widespread availability, applicability and inter-laboratory reproducibility (17). TE (FibroScan) appeared useful in this patient cohort and is a validated, user-friendly technique for assessing liver stiffness with excellent intra- and inter-observer agreement (18,19).
However, there are some vital points and unanswered questions that remain. First of all, the lack of histology for validation of the results for the non-invasive tests is a major limitation. While biopsy is certainly not suitable for liver screening due to its invasive, costly and potential life-threatening risks, it remains the reference method of choice, especially in an attempt to establish clinical guidelines with novel diagnostic tests such as in the present study of Strnad’s group (20). Secondly, TE has a relatively higher rate of diagnostic errors in obese patients (BMI ≥28 kg/m2), mostly in the M probe but also in the XL probe. When compared to the M, the XL probe used for obese patients measured lower stiffness values (by a median of 1.4 kPa) (21). 14.9% of the Pi*ZZ and 13.0% of non-carriers in the current study had a BMI ≥30 kg/m2. Another limiting factor is the lack of clear cutoff values for the classification of fibrosis using TE. These are currently lacking for AATD related liver disease, unless associations with histological fibrosis staging can be established. Nevertheless, TE is advantageous as it physically measures the liver stiffness directly, while the biomarker scores indirectly assess liver damage with several compounds. For instance, the HepaScore includes hyaluronic acid in its formula and high concentrations of it correlate with advanced liver fibrosis. However, there is no data available on hyaluronic acid and AATD associated liver disease, in fact it was shown to even be reduced in AATD lung tissue (22), which possibly makes it a confounding factor. Thus, a potential for error remains in utilizing indirect compound scores for establishing new guidelines. Importantly, the great number of patients included in this study now need to be monitored prospectively for complications of AATD induced liver disease (i.e. , progression to cirrhosis, decompensating events, liver transplantation, liver-related mortality) to evaluate the predictive value of the current results for patient-related endpoints.
A major finding of this large cohort study was that even asymptomatic Pi*ZZ carriers have signs of liver disease (steatosis, fibrosis). The study by Strnad and coworkers did not fully elucidate to which extent other risk factors for liver diseases (insulin resistance, metabolic syndrome, obesity, alcohol consumption) would drive the AATD related liver phenotype. In principle, Pi*ZZ could be viewed as a strong genetic determinant of liver injury, which raises the question on the relevance of the heterozygous Pi*MZ genotype for liver disease progression. Around 2.5% of the European population carries the Pi*MZ genotype, and the correlation between liver disease and heterozygous AATD was discussed previously (23). With regards to liver steatosis and non-alcoholic steatohepatitis, the genotypic and phenotypic correlation between Pi*ZZ (or Pi*MZ) and established genetic risk factors like PNPLA3, TM6SF2 and MBOAT7 would be of great interest. Taken together, the current study has brought substantial information on the liver disease burden of AATD and laid ground for future clinical trials to optimize assessment and counseling of AATD patients carrying the Pi*ZZ mutation.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 2012;185:246-59. [Crossref] [PubMed]
- DeMeo DL, Silverman EK. Alpha1-antitrypsin deficiency. 2: genetic aspects of alpha(1)-antitrypsin deficiency: phenotypes and genetic modifiers of emphysema risk. Thorax 2004;59:259-64. [Crossref] [PubMed]
- Sivasothy P, Dafforn TR, Gettins PG, et al. Pathogenic alpha 1-antitrypsin polymers are formed by reactive loop-beta-sheet A linkage. J Biol Chem 2000;275:33663-8. [Crossref] [PubMed]
- Rudnick DA, Perlmutter DH. Alpha-1-antitrypsin deficiency: a new paradigm for hepatocellular carcinoma in genetic liver disease. Hepatology 2005;42:514-21. [Crossref] [PubMed]
- Teckman JH, Qu D, Perlmutter DH. Molecular pathogenesis of liver disease in alpha1-antitrypsin deficiency. Hepatology 1996;24:1504-16. [PubMed]
- Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur Respir J 2017;50. [Crossref] [PubMed]
- Stoller JK. Chapter 9 - Alpha-1-Antitrypsin Deficiency: Epidemiological Studies and Other AATD Associated Diseases. In: Kalsheker N, Stockley R, editors. Alpha-1-antitrypsin Deficiency. Boston: Academic Press; 2017:133-58.
- Chu AS, Chopra KB, Perlmutter DH. Is severe progressive liver disease caused by alpha-1-antitrypsin deficiency more common in children or adults? Liver Transpl 2016;22:886-94. [Crossref] [PubMed]
- Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med 1976;294:1316-21. [Crossref] [PubMed]
- Sveger T. α1-Antitrypsin deficiency in early childhood. Pediatrics 1978;62:22-5. [PubMed]
- Bernspang E, Carlson J, Piitulainen E. The liver in 30-year-old individuals with alpha(1)-antitrypsin deficiency. Scand J Gastroenterol 2009;44:1349-55. [Crossref] [PubMed]
- Eriksson S. Alpha 1-antitrypsin deficiency and liver cirrhosis in adults. An analysis of 35 Swedish autopsied cases. Acta Med Scand 1987;221:461-7. [Crossref] [PubMed]
- Tanash HA, Nilsson PM, Nilsson J-Å, et al. Clinical course and prognosis of never-smokers with severe alpha-1-antitrypsin deficiency (PiZZ). Thorax 2008;63:1091-5. [Crossref] [PubMed]
- Seersholm N, Kok-Jensen A. Survival in relation to lung function and smoking cessation in patients with severe hereditary alpha 1-antitrypsin deficiency. Am J Respir Crit Care Med 1995;151:369-73. [Crossref] [PubMed]
- Hamesch K, Mandorfer M, Pereira VM, et al. Liver Fibrosis and Metabolic Alterations in Adults With alpha-1-antitrypsin Deficiency Caused by the Pi*ZZ Mutation. Gastroenterology 2019;157:705-19.e18. [Crossref] [PubMed]
- Teckman JH. Chapter 8 - Alpha-1-Antitrypsin Deficiency Liver Disease. In: Kalsheker N, Stockley R, editors. Alpha-1-antitrypsin Deficiency. Boston: Academic Press; 2017:117-31.
- Cales P, Veillon P, Konate A, et al. Reproducibility of blood tests of liver fibrosis in clinical practice. Clin Biochem 2008;41:10-8. [Crossref] [PubMed]
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007;56:968-73. [Crossref] [PubMed]
- Boursier J, Konate A, Gorea G, et al. Reproducibility of liver stiffness measurement by ultrasonographic elastometry. Clin Gastroenterol Hepatol 2008;6:1263-9. [Crossref] [PubMed]
- Piccinino F, Sagnelli E, Pasquale G, et al. Complications following percutaneous liver biopsy: A multicentre retrospective study on 68 276 biopsies. Journal of Hepatology 1986;2:165-73. [Crossref] [PubMed]
- Myers RP, Pomier-Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology 2012;55:199-208. [Crossref] [PubMed]
- Cantor J, Armand G, Turino G. Lung hyaluronan levels are decreased in alpha-1 antiprotease deficiency COPD. Respir Med 2015;109:656-9. [Crossref] [PubMed]
- Kok KF, Wahab PJ, Houwen RH, et al. Heterozygous alpha-I antitrypsin deficiency as a co-factor in the development of chronic liver disease: a review. Neth J Med 2007;65:160-6. [PubMed]
Cite this article as: Roohani S, Tacke F. Non-invasive assessment for alpha-1 antitrypsin deficiency-associated liver disease: new insights on steatosis and fibrosis in Pi*ZZ carriers. Transl Gastroenterol Hepatol 2019;4:82.


