
Contrast enhanced ultrasound: comparing a novel modality to MRI to assess for bowel disease in pediatric Crohn’s patients
Introduction
The prevalence of Crohn’s disease (CD) is highest in North America, with 145–199 cases per 100,000 people (1). It often results in focal, asymmetrical, and transmural bowel inflammation. Assessing for bowel wall involvement and extent of disease is critical, as it can portend the severity of illness. There is no literature comparing magnetic resonance imaging (MRI) and contrast enhanced ultrasound (CEUS) to qualitatively assess for bowel disease in children. A small study in 2015 assessed 28 children (mean age 14 years) with greyscale ultrasound, who were known to have bowel wall inflammation on MRI. Greyscale ultrasound was found to have a sensitivity of 55% and specificity of 57% (2). In the adult literature, a study performed in February 2017 compared the presence of bowel disease on MRI versus CEUS in adult patients with severe CD and found a strong correlation between inflammation between the two modalities (P<0.001) (3). Our study aimed to explore a novel imaging technique and corroborate its validity to assess for distal small bowel disease in pediatric patients, thereby providing an alternative imaging modality.
In the past decade, MRI has come to revolutionize diagnostic algorithms in diagnosis and management of CD as it allows visualization of mural thickening, fibrosis, perianal disease and abscesses without radiation as well as subtle secondary signs of bowel inflammation, including pericolonic fat stranding. Despite the advantages of MRI examinations, limitations include the use of oral contrast agents for colonic luminal distension, which can be challenging for children, high cost of MRI, length of the procedure and anesthesia in small children. In comparison, a high quality abdominal ultrasound can assess for bowel wall thickness, vascularization, abdominal free fluid, mesenteric lymph nodes, stenosis, fistulas, and abscesses, without the need for oral contrast or anesthesia (4). Although ultrasound has a lower tissue contrast than MRI, it has a higher spatial resolution which allows for detailed visualization of different layers of the bowel wall. When emphasized with microbubble contrast, which consists of proteins, lipids, and biopolymers that rapidly distribute in the vessels, there may be the potential to accentuate visualization of the bowel wall, and assess for the presence of mucosal disruption, pericolonic fat, and intramural small abscesses, all of which can be a sign of more severe active disease (5). This enhancement of the bowel wall is important as studies have shown that mural thickness of the combined mucosa and submucosa greater than 3 mm, when it is able to be visualized on ultrasound, has a sensitivity and specificity of 88% and 95% respectively for CD and active inflammation (6,7). Thus, this may be the most reliable indicator of small bowel disease. In addition, early neovascularization of the bowel wall, a pathological change seen in CD, is enhanced by microbubbles, and therefore can accentuate areas of early inflammation.
Our goal therefore is to demonstrate both the feasibility and reliability of this novel radiographic technique to evaluate for distal small bowel disease in CD as compared to MRI.
Methods
Inclusion
All patients with diagnosed or suspected diagnosis of CD, less than 21 years of age, who merited imaging with MRI based on physician criteria as below, to assess for distal small bowel disease.
Exclusion
None. If coordinating a same day CEUS delayed patient care in anyway, then patients were not included in the study, and priority was given to obtaining an MRI.
Data, including age, gender, inpatient/outpatient status, reason for obtaining imaging, and ultimate diagnosis were obtained. Congruency of enhancement pattern between MRI and CEUS was recorded for each patient.
After assessment by a board-certified pediatric gastroenterologist at Lucile Packard Children’s Hospital Stanford, inpatient and outpatient pediatric patients who had clinical suspicion or diagnosis of CD, based on clinical criteria of abdominal pain, weight loss, bloody stools, fevers, diarrhea, or surveillance needs, who merited further imaging with a contrast based MRI (T1- and T2-weighted images) underwent a CEUS, ideally on the same day. Trained sonographers in conjunction with a board-certified pediatric radiologist at Lucile Packard Children’s Hospital with 15 years of experience, performed and interpreted the CEUS. The patients were initially scanned in grayscale to identify the region of bowel wall thickening or inflammation. The bowel was evaluated on grayscale images for signs of inflammation. Inflammation was noted when there was thickening of the mucosa or submucosa, loss of normal bowel stratification, disruption of the muscularis propria, presence of intramural abscesses, increased and hyperechoic fat. If inflammation was not apparent, images of the terminal ileum were obtained. After the area of interest was localized, contrast (sulfur hexafluoride lipid-A microspheres, Lumason®, Bracco) was injected at a dose of 0.03 mL/kg. Ultrasound was performed with Philips, GE and Siemens machines, with probes ranging from 6–18 MHz. Degrees of enhancement of the bowel wall and inflammatory fat was assessed over a period of 5 minutes post contrast injection.
We then compared this with findings of inflammation as seen on standard MRI (3T Discovery 750, GE Healthcare, T1- and T2-weighted images with and without contrast), which was read by a different radiologist to avoid bias. MRI was performed in patients after they were asked to drink biphasic oral contrast, which presents as hypointense on T1-weighted images and hyperintense on T2-weighted sequences. The sequences for our examination included a coronal single shot T2-weighted sequence (SSFSE), cinematic thick slab coronal balanced gradient echo to evaluate peristalsis (Coronal FIESTA Dynamic), multiphase coronal T1-weighted fat suppressed images with contrast injection, and axial T2-weighted fat suppressed sequence of the abdomen and pelvis (FIESTA). Disease features on MRI indicative of enhancement included increased wall thickness, high T2 signal intensity and bowel wall transmural hyper-enhancement. We also were able to assess for pericolonic inflammation, which on MRI presents as an ill-defined region of T2 hyperintense signal and iso-intense T1 signal in the fat surrounding a bowel loop, and mucosal disruption, which is the loss of stratification between continuous areas of bowel wall. The CEUS in most cases were done on the same day as the MRI, however given occasional difficulties of scheduling patients on the same day, or time restraints on behalf of patients, two patients had up to a week discrepancy between the two studies.
Results
Over the time period from April 2018 to January 2019 we enrolled a total of 20 patients for this study. These patients had an average age of age of 14.2 years of age, with 9 females and 11 males recruited (Table 1). Eighty-five percent of patients (n=17) had concordance of bowel enhancement, 6 without enhancement on either modality, and 11 with enhancement on both modalities (Table 2). The remaining 3 patients had discordant findings, with inflammations seen on CEUS but not on MRI. Excluding our patients without enhancement on either modality (#1–6), of the remaining 14 patients, all with known CD, 71% (n=10) of patients had signs of mucosal disruption on CEUS, whereas only 21% (n=3) had signs of mucosal disruption on MRI. Radiological evidence of inflammatory fat stranding, a characteristic seen namely in CD, was observed in 93% of patients (n=13).
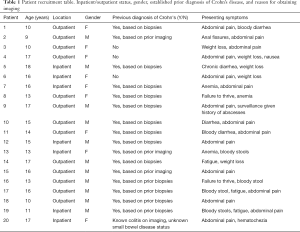
Full table
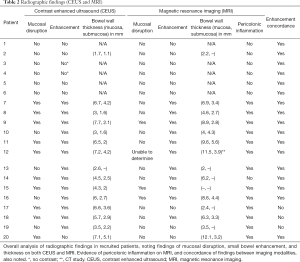
Full table
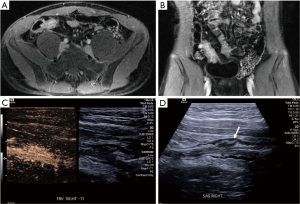
Six patients (#1–6) recruited in the study, three with a known diagnosis of CD, had both normal MRI and ultrasound imaging studies. However, two of these patients had traditional greyscale imaging without contrast, given the greyscale was overwhelmingly normal in appearance. Eleven patients who had a known diagnosis of CD, with previously known small bowel disease, had findings of small bowel disease on both CEUS and MRI (Figure 1). One of these patients had a CT done in lieu of an MRI.
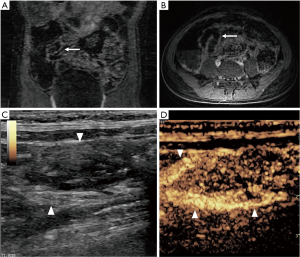
There were three patients with known CD who had normal MRI studies without significant enhancement; however, all three had prominent enhancing bowel loops on CEUS (Cases #17–19). Although patient #18 had very subtle enhancement on MRI, there was very prominent enhancement on the CEUS, and in essence this could be viewed as discordant (Figure 2). Patient #20 in this study had an unknown diagnosis of CD, with colitis on biopsy, and underwent both a magnetic resonance enterography (MRE) and CEUS, both revealing colonic disease without small bowel enhancement; however the CEUS noted significant pericolonic fat stranding around the colon, not previously noted on MRI.
Discussion
In our study, we demonstrate the feasibility and reliability of CEUS for CD in children. All the patients who had findings of small bowel inflammation on cross sectional studies (MRI in 11 patients and one patient on CT) had evidence inflammation on CEUS, making the sensitivity of our study 100%. Two patients in this study had findings of enhancement on CEUS which was not seen MRI; and one patient although had showed enhancement of the small bowel on both studies, the level of enhancement on CEUS was markedly more pronounced compared to the subtle findings on MRI. One patient had known colitis and was undergoing imaging to evaluate for small bowel disease to distinguish between UC and CD, and had only colonic involvement on both modalities. However, this patient did have notable inflammatory fat around the colon on CEUS, giving supportive evidence for CD. Although this was not our specific intention, pericolonic fat is mainly seen in CD, and not in ulcerative colitis, and helped to make a salient diagnosis for this patient. Due to the high resolution of ultrasound, subtle changes in mucosal and submucosal thickening could be detected on ultrasound while not visible on MRI.
In terms of the overall ability to highlight areas of inflammation in general, CEUS was able to show enhancement of the submucosa and mucosa of the bowel in all 14 of our patients with CD, and pick up signs of mucosal disruption in nearly 71% of these patients. Obviously, the ability to evaluate these objective findings is dependent on the bowel segment that is visualized, but given we were able to see a depth of enhanced bowel greater than 3 mm (indicative of inflammation) in all of our patients, this was very reassuring, especially given that our controls had no enhancement to any degree.
From a safety standpoint, CEUS has a proven track record. Thirty-four subjects ranging in age from 8 months to 20.7 years underwent 134 CEUS procedures with no significant side effects, and all tolerated the procedure well (8). An additional study examined the records of 305 pediatric patients aged 1 month to 18 years, who were undergoing CEUS for a variety of etiologies, including renal, vascular, trauma, and liver concerns, and none had adverse reactions related to CEUS (9). Some of these patients also had MRI studies done synchronously with their CEUS, similar to our study design, and none had any adverse outcomes. Our IRB has also reviewed the safety merits of microbubble contrast, in conjunction with MRI contrast, and deemed there was no excessive burden of risk placed patients, and we had their written approval prior to enrolling patients.
The discretion of enrollment was completely up to the patient, and if some caregivers did not want to participate, they were excluded, which may have biased our patient selection. Furthermore, we only consented patients who had an MRI ordered by their provider, and given that providers have different thresholds and reasons for ordering MRI studies, this could also have biased on our population sample. Given that we wanted to demonstrate findings, and prove the feasibility of this study in a relatively short time frame, we did not perform this study on patients if we felt there was a strong suspicion of functional abdominal pain, and therefore we enrolled patients who would likely have small bowel inflammation.
We realize that though we could quantify the degree of inflammation on CEUS, we were not able to quantify the extent of bowel disease on CEUS, given that ultrasound has a small field of view compared to MRI, which is one of the major limitations of ultrasounds. Therefore, due to a lack of a reliable quantitative, post-processing tool to evaluate quantitatively for degree of bowel enhancement, we sought to obtain namely qualitative as opposed to quantitative data for the purposes of our study. We are also aware that CEUS can evaluate additional radiological findings such as fat stranding (as noted in one patient), abscesses, strictures, fistulas, etc.; however given the enrollment size of our initial group, we were not able to evaluate these in further depth, but this can be a consideration in future studies.
In addition, given our radiological ultrasounds machines and contrast software was constantly upgraded, we used machines from three different vendors, and therefore the different machines may have subtle differences in their abilities to assess for enhancement. Furthermore, given ultrasound is limited in some degree by the technician and by the limited field of view; unlike the case of an MRI, we may not have been able to visualize a particular loop of bowel of interest due to the smaller field of view.
An additional consideration is that for the purposes of this study, we assumed that the MRI was the gold standard of inflammation, wherein all practically, the gold standard would be actual small bowel biopsies. Although nearly all patients had prior evidence of small bowel inflammation histologically, many of these had these biopsies chronologically separated from our study (i.e., biopsy several years ago for diagnosis of CD) and therefore we could not corroborate either MRI or CEUS with a true gold standard of current and real-time small bowel inflammation. Furthermore, in the cases where CEUS showed inflammation but MRI was normal, we had no gold standard to corroborate this was not a false positive result. We also aimed to have as many studies done temporally within 24 hours of each other, but due to scheduling and patient time constraints two patients may have had up to a week delay between the MRI and CEUS, and theoretically they could have had a change in disease burden, either for better or worse, during this time, which may have biased our results.
Despite the limitations of the study, in conclusion, we illustrated that CEUS can accurately detect and assess for small bowel inflammation, particularly in patients with CD. Feasibility of CEUS would help in reducing the limitations of performing MRE in pediatric patients; mainly necessity of oral contrast, time of exam, cost and possibility of sedation. However, more structured, controlled, and larger population sizes would be required to truly potentiate this imaging modality as a valid and recognizable modality. Overall, there may be a significant role for using CEUS in assessing for pediatric small bowel inflammation in CD.
Acknowledgments
Funding provided by a grant through Stanford, Child Health Research Institute, Stanford University School of Medicine, Stanford, CA, USA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical Approval: Stanford IRB Approval 42949. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Bhatnagar G, Von Stempel C, Halligan S, et al. Utility of MR enterography and ultrasound for the investigation of small bowel Crohn's disease. J Magn Reson Imaging 2017;45:1573-88. [Crossref] [PubMed]
- Horjus Talabur Horje CS, Bruijnen R, Roovers L, et al. Contrast Enhanced Abdominal Ultrasound in the Assessment of Ileal Inflammation in Crohn's Disease: A Comparison with MR Enterography. PLoS One 2015;10:e0136105. [Crossref] [PubMed]
- Wilkens R, Peters DA, Nielsen AH, et al. Dynamic Contrast-Enhanced Magnetic Resonance Enterography and Dynamic Contrast-Enhanced Ultrasonography in Crohn's Disease: An Observational Comparison Study. Ultrasound Int Open 2017;3:E13-24. [Crossref] [PubMed]
- Conti CB, Giunta M, Gridavilla D, et al. Role of Bowel Ultrasound in the Diagnosis and Follow-up of Patients with Crohn's Disease. Ultrasound Med Biol 2017;43:725-34. [Crossref] [PubMed]
- Gee MS, Harisinghani MG. MRI in patients with inflammatory bowel disease. J Magn Reson Imaging 2011;33:527-34. [Crossref] [PubMed]
- Parente F, Greco S, Molteni M, et al. Modern imaging of Crohn's disease using bowel ultrasound. Inflamm Bowel Dis 2004;10:452-61. [Crossref] [PubMed]
- Horsthuis K, Stokkers PC, Stoker J. Detection of inflammatory bowel disease: diagnostic performance of cross-sectional imaging modalities. Abdom Imaging 2008;33:407-16. [Crossref] [PubMed]
- Coleman JL, Navid F, Furman WL, et al. Safety of ultrasound contrast agents in the pediatric oncologic population: a single-institution experience. AJR Am J Roentgenol 2014;202:966-70. [Crossref] [PubMed]
- Yusuf GT, Sellars ME, Deganello A, et al. Retrospective Analysis of the Safety and Cost Implications of Pediatric Contrast-Enhanced Ultrasound at a Single Center. AJR Am J Roentgenol 2017;208:446-52. [Crossref] [PubMed]
Cite this article as: Mudambi K, Sandberg J, Bass D, Rubesova E. Contrast enhanced ultrasound: comparing a novel modality to MRI to assess for bowel disease in pediatric Crohn’s patients. Transl Gastroenterol Hepatol 2020;5:13.


