Glycoprotein non-metastatic melanoma B expression after hepatic ischemia reperfusion and the effect of silibinin
Introduction
Hepatic blood inflow occlusion is often applied during complex surgical operations such as transplantation or hepatectomy, with the intent to restrict blood loss. However, this also subjects the liver to ischemia-reperfusion (I/R) injury, a type of cellular damage initiated during ischemia by low-oxygen supply and deteriorated during reperfusion due to inflammatory and oxidative phenomena (1). Hepatic I/R has unfavorable sequelae on remote organs such as brain, adrenals, lungs and kidneys, through partly known mechanisms. Those include transient portal hypertension and release of inflammatory cytokines (2). Hepatic I/R has significant sequelae such as renal failure, acute lung injury, multiorgan failure and even death. Considering the above, hepatic I/R is a significant research area, with modern research focusing on events at cellular level.
Glycoprotein non-metastatic melanoma B (GPNMB) was discovered by Weterman et al. as a transmembrane glycoprotein expressed in melanoma cells with low metastatic potential (3). In 2001, Safadi et al. (4) described GPNMB as a homologous gene in rats with osteopetrosis Since its discovery, many researchers have focused on its role in oncogenesis (5-7) while others have focused on the GPNMB complex role as an inflammation mediator (7,8). Relevant clinical models focusing on GPNMB include cirrhosis, non-alcoholic fatty liver disease and hepatic I/R (9-11).
Silibinin is the major active compound of sylimarin, the extract of milk thistle seeds (Silybum marianum) (12) with hepatoprotective properties, acting as an antioxidant free radical scavenger and an inhibitor of lipid peroxidation (12-15). We recently demonstrated the hepatoprotective, nephroprotective and anti-inflammatory effect of silibinin under hepatic I/R conditions (16).
In the present study, we further explore the hepatoprotective and nephroprotective effect of silibinin under hepatic I/R conditions. More specifically, we study the GPNMB expression at various time points in liver and kidney tissues, using a liver I/R rat model, examining both direct and remote I/R effects, and the protective role of silibinin administration, with a view to explore its potential applicability in liver surgery. To the best of our knowledge, GPNMB expression during I/R injury has not been studied in the literature, either in hepatic or kidney tissue.
Methods
Animal handling
Experiments were performed in the animal facility of the Center of Clinical, Experimental Surgery and Translational Research of the Biomedical Research Foundation of the Academy of Athens. The facility is registered as a “breeding” and “experimental” facility according to the Greek Presidential Decree 56/2013, which harmonizes national legislation with the European Community Directive 2010/63 on the Protection of Animals Used for Experimental and Other Scientific Purposes. All animal handling and experimental procedures were conducted according to the National Research Council’s Guide for the Care and Use of Laboratory Animals, and Directive 86/609 of the European Union. The study was approved by the Veterinary Authorities of the Region of Athens, Greece (583/05-02-2015).
Experimental I/R protocol
Sixty-three Wistar male rats (age 13.25±4.40 weeks) were assigned into three groups, namely sham (n=7), control (C) (n=28) and silibinin (Si) (n=28). In the sham group, no intervention apart from open-close laparotomy was performed. In the control group, 45 min of ischemia were applied through occlusion of the hepatoduodenal ligament. Reperfusion followed as discussed below. In the Si group, silibinin was administered intravenously, as a lyophilized SLB-HP-β-CD complex reconstituted in water for injection immediately before reperfusion. Preparation of SLB-HP-β-CD lyophilized product was performed as previously described using a freeze-drying procedure and the neutralization method (17,18). Briefly, 0.300 g of silibinin (MW =482.44) and 1.860 g of HP-β-CD (MW =1540) (both from Sigma Aldrich, Steinheim, Germany, purity >99%) were transferred in a 300 mL volumetric flask, and suspended with 200 mL of water (triple-de-ionized water from Millipore was used for all preparations). Small amounts of ammonium hydroxide were then added under continuous stirring and pH monitoring, until complete dissolution and pH adjustment to a value between 9 and 10 was obtained. The resulting solution at a molar ratio of 1:2 was thereafter freeze-dried using a Kryodos-50 model Telstar lyophilizer.
Control and silibinin groups were equally subdivided into 4 sub-groups each representing one sampling time point according to the duration of allowed reperfusion, namely control sub-groups C60, C120, C180 and C240, and silibinin sub-groups Si60, Si120, Si180 and Si240, for 60, 120, 180 and 240 min of euthanasia after reperfusion, respectively. Euthanasia was followed by liver and kidney tissue sample harvesting. The experiment was performed under general anesthesia with isoflurane.
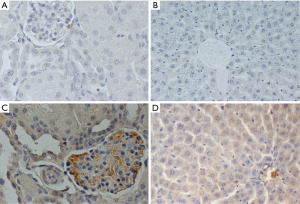
Tissue specimens were fixed in formalin solution, neutral buffered 10% and embedded in paraffin according to standard histological protocol. Four microns unstained sections (4 µm) of representative blocks from each case were deparaffinized, rehydrated, and treated with 0.3% H2O2 at room temperature for 15 min in order to block the bioactivity of endogenous peroxidase, and then were immunostained by the Super Sensitive Onestep Polymer-HRP Detection System (QD 630-XAKE, Biogenex). Slides were incubated for 60 min with primary anti–GPNMB rabbit polyclonal antibody, in dilution 1:100 (Biorbyt, UK). The positive expression of antibodies was determined by counting the number of stained cells (cytoplasmic or membrane localization). The average labeling index of their expression was assessed according to the proportion of positive cells. The immunohistochemistry evaluation was blinded to the examined subgroup or examined time point. The results of antibody expression, which was mostly cytoplasmic (only 8 samples had cytoplasmic and membrane expression simultaneously), were graded negative (0) for <10% of stained cells, low (I) for >20% and <25% of stained cells, moderate (II) for >25% and <50% of stained cells, high (III) for >50% of stained cells. Intensity was not scored separately. Sections with <10% stained cells were evaluated as negative (0) (16).
Statistical data analysis was performed using SPPS (v21). Immunohistochemical data were considered as ordinal, and thus, tests used were the linear-by-linear trend and Chi-square test. P value <0.05 was considered statistically significant.
Results
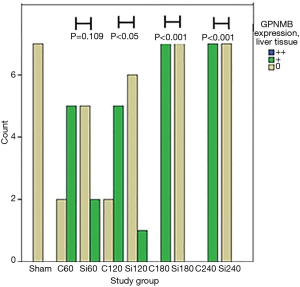
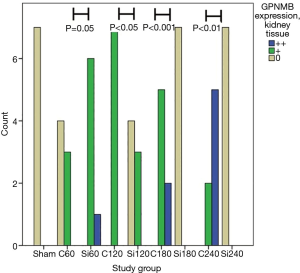
GPNMB expression dataset for liver and kidney tissue are presented in Table 1. GPNMB expression was absent in sham groups both in liver and kidney tissues. Representative histopathology results are shown in Figure 1 both for the control and silibinin groups. Statistical analysis of the results is shown in Tables 2 and 3.

Full table
Full table

Full table
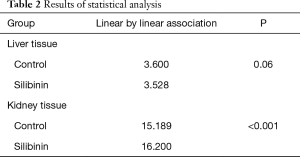
A trend of decreasing GPNMB expression throughout the experiment was found for the silibinin groups in liver tissue. Before the end of the experiment (180 min), the GPNMB expression was 0 for all animals in the silibinin groups and was maintained so until the end of the experiment. This decreasing trend in silibinin groups in liver tissue was statistically indicative (P=0.06). In the control group, in kidney tissue, a statistically significant trend of increasing GPNMB expression was found from the 60 min to the 240 min time point after reperfusion. A statistically significant trend of decreasing GPNMB expressions in kidney tissue was found in the silibinin groups (Table 2).
A comparison between corresponding control and silibinin groups is shown (Table 3; Figures 2,3). Statistically significant differences were found between all groups, except for 60 min group in liver tissue. The results indicate that silibinin inhibits the expression of GPNMB in the liver and kidney tissues in the rat liver I/R model. Considering the role of GPNMB as inflammation mediator, the results indicate the potential protective role of silibinin on both the liver and the kidney.
Discussion
GPNMB was discovered as an onco-suppressor gene due to its expression at melanoma cells of low metastatic potential. Soon after, Loging et al. (19) challenged this hypothesis by reporting high GPNMB expression in glioblastoma cells of high metastatic potential. Onaga et al. (20) reported GPNMB overexpression in hepatoma cells in human and rat tissues, further amplifying its role as an oncogene. However even today the role of GNPMB in oncogenesis needs further investigation. Solid data exist for the role of GPNMB in human breast cancer with its overexpression signifying a more malignant phenotype (21). Clinical research has led to the development of Glembatumumab Vedotin, a conjugated GPNMB-auristatin E antibody against breast cancer (22).
Silibinin is the major active compound of sylimarin, the extract of milk thistle seeds (Silybum marianum) (12). We recently demonstrated the hepatoprotective, nephroprotective and anti-inflammatory effect of silibinin under hepatic I/R conditions on the same rat model, through the reduced expression of specific biomarkers in liver (Fas/FasL, HMGB-1, CD45) and kidney (TNF-α and M30) tissues, as well as by pharmacokinetic results showing higher liver accumulation and slower plasma elimination of silibinin in ischemic animals (16,23).
Research has also focused on GPNMB role on inflammation, especially at liver injury. GPNMB is regulated by various stimulants and interacts with metalloproteinases, platelet derived growth factor and tissue inhibitor metalloproteinases, participating in tissue remodeling and fibrosis (9,10). Further research has shown that GPNMB acts as a phagosome protein and is important for cellular debris scavenging and tissue remodeling (24).
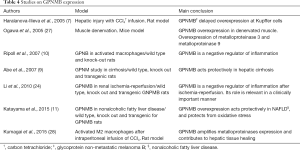
GPNMB expression has been studied under different experimental scenarios. Haralanova-Ilieva et al. (7) studied GPNMB expression in an experimental model of liver injury after intraperitoneal infusion of carbon tetrachloride (CCl4). They found significant expression of GPNMB in liver tissue. GPNMB was expressed by Kupffer cells and macrophages that were either native in liver tissue or sequestrated from circulation. Furthermore, they studied GPNMB kinetics and found that increased expression starts 4 h after liver injury, peaks at 48 h and starts depleting after 168 h. Abe et al. (9) studied its expression in cirrhotic rat liver in wild type, knock out and transgenic mice. They found that transgenic mice were protected from hepatic injury. Protection seemed to act at the hepatic stellate cells level since: (I) expressions of collagen I, platelet derived growth factor α and type 1 and 2 of tissue inhibitors of metalloproteinases were diminished; and (II) no changes in biochemical values of the other groups were observed. Katayama et al. (11) studied osteoactivin and GPNMB expression in non-alcoholic fatty liver disease in wild type, knock out and transgenic mice. They found that GPNMB expression depends on many inflammation regulators, including bone morphogenic protein B, receptor activated nuclear factor κ ligand and colony stimulating factor. Furthermore, they showed increased soluble GPNMB in human serum of patients with non-alcoholic fatty liver disease and type 2 diabetes.
GPNMB expression has been studied in models of renal damage. In an experimental model similar to ours, Li et al. (24) studied GPNMB expression in a direct kidney I/R model in wild-type, knock-out and transgenic mice. Macrophages in knock-out mice were defective in scavenging apoptotic material and consequently in repairing renal tubules after I/R injury. Knock-out mice developed renal failure and experienced 85% increase in mortality following bilateral ischemic kidney injury. They also studied GPNMB kinetics, defining that it contributes in phagosome function. Similarly Zhou et al also found overexpression of GPNMB at rats kidney tissues subjected to acute ischemic injury (25).
Studies of GPNMB in humans are limited. Pahl et al. (26) found increased levels of soluble GPNMB in dialysis patients. According to them, GPNMB increase is attributed on increased macrophages—monocytes transformation due to chronic inflammation caused by dialysis and chronic uremia. A summary of studies on GPNMB and inflammation can be found in Table 4.
Full table
The aim of our study was to give further insight to our previous work (16,23) where the hepatoprotective and nephroprotective effect of silibinin under hepatic I/R conditions was demonstrated through its effect on the tissue expression of various I/R related biomarkers, such as Fas/FasL, HMGB-1, CD45, TNF-α and M30. To this end, the GPNMB expression at various time points after reperfusion was studied in liver and kidney tissues, for the first time, in a rat model subjected to liver I/R injury. The protective role of intravenous silibinin administration on both direct and remote organs after I/R adverse effects is shown through the amelioration of the expression of an additional marker, GPNMB, thus making the use of silibinin promising in hepatic surgery.
In conclusion, the immunohistological results of our rat model indicate that GPNMB is overexpressed during hepatic injury in both the liver and the kidneys. A progressive and time–dependent increase in GPNMB expression during I/R injury was observed in kidney tissue. Furthermore, we observed the protective action of silibinin in liver injury through significant decrease in GPNMB expression. The silibinin protective effect during hepatic I/R does not only act in a local but also in a remote manner, as it expands to kidneys.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work areappropriately investigated and resolved. All animal experiments were performed in the animal facility of the Center of Clinical, Experimental Surgery and Translational Research of the Biomedical Research Foundation of the Academy of Athens, and followed the relevant European and national laws and guidelines for the ethical treatment of experimental animals. The study was approved by the Veterinary Authorities of the Region of Athens, Greece (583/05-02-2015).
References
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol 2003;284:G15-26. [Crossref] [PubMed]
- Nastos C, Kalimeris K, Papoutsidakis N, et al. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev 2014;2014:906965.
- Weterman MA, Ajubi N, van Dinter IM, et al. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer 1995;60:73-81. [Crossref] [PubMed]
- Safadi FF, Xu J, Smock SL, et al. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J Cell Biochem 2001;84:12-26. [Crossref] [PubMed]
- Maric G, Rose AA, Annis MG, et al. Glycoprotein non-metastatic b (GPNMB): A metastatic mediator and emerging therapeutic target in cancer. Onco Targets Ther 2013;6:839-52. [PubMed]
- Kuan CT, Wakiya K, Dowell JM, et al. Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme. Clin Cancer Res 2006;12:1970-82. [Crossref] [PubMed]
- Haralanova-Ilieva B, Ramadori G, Armbrust T. Expression of osteoactivin in rat and human liver and isolated rat liver cells. J Hepatol 2005;42:565-72. [Crossref] [PubMed]
- Owen TA, Smock SL, Prakash S, et al. Identification and characterization of the genes encoding human and mouse osteoactivin. Crit Rev Eukaryot Gene Expr 2003;13:205-20. [Crossref] [PubMed]
- Abe H, Uto H, Takami Y, et al. Transgenic expression of osteoactivin in the liver attenuates hepatic fibrosis in rats. Biochem Biophys Res Commun 2007;356:610-5. [Crossref] [PubMed]
- Ripoll VM, Irvine KM, Ravasi T, et al. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol 2007;178:6557-66. [Crossref] [PubMed]
- Katayama A, Nakatsuka A, Eguchi J, et al. Beneficial impact of Gpnmb and its significance as a biomarker in nonalcoholic steatohepatitis. Sci Rep 2015;5:16920. [Crossref] [PubMed]
- Tsai MJ, Liao JF, Lin DY, et al. Silymarin protects spinal cord and cortical cells against oxidative stress and lipopolysaccharide stimulation. Neurochem Int 2010;57:867-75. [Crossref] [PubMed]
- Woo SM, Min KJ, Kim S, et al. Silibinin induces apoptosis of HT29 colon carcinoma cells through early growth response-1 (EGR-1)-mediated non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) up-regulation. Chem Biol Interact 2014;211:36-43. [Crossref] [PubMed]
- Gazák R, Walterová D, Kren V. Silybin and silymarin--new and emerging applications in medicine. Curr Med Chem 2007;14:315-38. [Crossref] [PubMed]
- Surai PF. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants (Basel) 2015;4:204-47. [Crossref] [PubMed]
- Tsaroucha AK, Valsami G, Kostomitsopoulos N, et al. Silibinin Effect on Fas/FasL, HMGB1, and CD45 Expressions in a Rat Model Subjected to Liver Ischemia-Reperfusion Injury. J Invest Surg 2018;31:491-502. [Crossref] [PubMed]
- Kellici TF, Ntountaniotis D, Leonis G, et al. Investigation of the interactions of silibinin with 2-hydroxypropyl-β-cyclodextrin through biophysical techniques and computational methods. Mol Pharm 2015;12:954-65. [Crossref] [PubMed]
- Christodoulou E, Kechagia IA, Tzimas S, et al. Serum and tissue pharmacokinetics of silibinin after per os and i.v. administration to mice as a HP-β-CD lyophilized product. Int J Pharm 2015;493:366-73. [Crossref] [PubMed]
- Loging WT, Lal A, Siu IM, et al. Identifying potential tumor markers and antigens by database mining and rapid expression screening. Genome Res 2000;10:1393-402. [Crossref] [PubMed]
- Onaga M, Ido A, Hasuike S, Uto H, et al. Osteoactivin expressed during cirrhosis development in rats fed a choline-deficient, L-amino acid-defined diet, accelerates motility of hepatoma cells. J Hepatol 2003;39:779-85. [Crossref] [PubMed]
- Rose AAN, Annis MG, Dong Z, et al. ADAM10 releases a soluble form of the GPNMB/Osteoactivin extracellular domain with angiogenic properties. PLoS One 2010;5:e12093. [Crossref] [PubMed]
- Saleh M, Bendell J, Rose A, et al. Correlation of GPNMB expression with outcome in breast cancer (BC) patients treated with the antibody-drug conjugate (ADC), CDX-011 (CR011-vcMMAE. J Clin Oncol 2010;28:1095. [Crossref]
- Kyriakopoulos G, Tsaroucha AK, Valsami G, et al. Silibinin Improves TNF-α and M30 Expression and Histological Parameters in Rat Kidneys After Hepatic Ischemia/Reperfusion. J Invest Surg 2018;31:201-9. [Crossref] [PubMed]
- Li B, Castano AP, Hudson TE, Nowlin BT, et al. The melanoma-associated transmembrane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair. FASEB J 2010;24:4767-81. [Crossref] [PubMed]
- Zhou L, Zhuo H, Ouyang H, et al. Glycoprotein non-metastatic melanoma protein b (Gpnmb) is highly expressed in macrophages of acute injured kidney and promotes M2 macrophages polarization. Cell Immunol 2017;316:53-60. [Crossref] [PubMed]
- Pahl MV, Vaziri ND, Yuan J, et al. Upregulation of monocyte/macrophage HGFIN (Gpnmb/Osteoactivin) expression in end-stage renal disease. Clin J Am Soc Nephrol 2010;5:56-61. [Crossref] [PubMed]
- Ogawa T, Nikawa T, Furochi H, et al. Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am J Physiol Cell Physiol 2005;289:C697-707. [Crossref] [PubMed]
- Kumagai K, Tabu K, Sasaki F, et al. Glycoprotein Nonmetastatic Melanoma B (Gpnmb)-Positive Macrophages Contribute to the Balance between Fibrosis and Fibrolysis during the Repair of Acute Liver Injury in Mice. PLoS One 2015;10:e0143413. [Crossref] [PubMed]
Cite this article as: Michalinos A, Tsaroucha AK, Lambropoulou M, Schizas D, Valsami G, Kostomitsopoulos N, Pitiakoudis MS, Simopoulos CE. Glycoprotein non-metastatic melanoma B expression after hepatic ischemia reperfusion and the effect of silibinin. Transl Gastroenterol Hepatol 2020;5:7.