Opioid use is associated with incomplete capsule endoscopy examinations: a systematic review and meta-analysis
Introduction
Wireless video capsule endoscopy (CE) is a non-invasive imaging method designed for evaluating the small intestine for many gastrointestinal (GI) disease processes (1). CE is predominantly used in evaluating obscure gastrointestinal bleeding (OGIB), detecting small bowel lesions, and examining patients with Crohn’s disease (1).
Since approved by the United States Food and Drug Administration (FDA) in 2001, CE technology has continued to evolve. Multiple studies have shown CE to provide superior diagnostic yield when compared to push enteroscopy and computed tomography angiography for OGIB (1,2). It has been estimated that CE has an overall pathology detection rate of approximately 60% (3).
While primarily being utilized for the evaluation of suspected small bowel bleeding in adults, CE is also used for the diagnosis of small intestinal tumors and Crohn’s disease. Furthermore, CE is now being utilized to evaluate a myriad of other small bowel pathologies such as non-steroidal anti-inflammatory drug (NSAID) enteropathy, celiac disease, and polyposis syndromes (4-6).
CE is regarded as a generally safe procedure with no reported cases of death associated with this procedure (7,8). However, capsule retention and incomplete CE examinations remain two barriers hindering its clinical utility. Capsule retention affects 1.4% of the tested patients with 59% of these patients requiring surgical intervention to remove the capsule (3). The highest rates of retention documented are in patients being evaluated for subacute small bowel obstruction (10–20% retention rate) and patients with known small bowel tumors (10–25% retention rate) (9-12). Furthermore, capsule retention can reveal the location of the culprit pathology and guide surgical intervention (13).
Incomplete examination defined as failure to reach the cecum within the battery lifespan, is the result of transient capsule retention along the GI tract, with a reported completion rate of 83.5% for the standard 8-hour device as of 2010 (3). Incomplete examination may result in inadequate diagnosis of small bowel pathologies and can lead to delayed intervention or repeated studies. Ultimately, incomplete examination inconveniences the patient and drives up costs to the medical system (2).
For nearly as long as the medical community has been aware of the analgesic effects of opioids, it has used opioids to treat diarrhea (14). Starting in 1917 with Trendelenburg’s discovery that morphine has an inhibitory effect on GI peristalsis, the field of electrophysiology has been critical in demonstrating the impact opiates have on the enteric nervous system (15,16). Today, the medical community builds on the discovered therapeutic benefits of opioids as patients are regularly prescribed loperamide for the treatment of diarrhea (17). Additionally, methylnaltrexone, a drug often used to treat opioid-induced constipation, was recently approved by the FDA and deemed to be safe and effective for the treatment of irritable bowel syndrome-diarrhea subtype in adults (18).
The enteric nervous system, composed of the myenteric and submucosal plexus, contain a high density of opioid receptors that regulate motility and secretions (19). Activation of the µ-opioid receptors specifically within the gut wall coordinates GI motility by inhibiting neural pathways within the enteric nervous system (20). Inhibited excitatory neural pathways lead to weaker peristalsis. On the other hand, inhibited inhibitory neural pathways lead to heightened GI muscle tone and motionless motor patterns (21). Together, inhibition of both excitatory and inhibitory pathways by activation of µ-opioid receptors cause delayed gastric emptying and increased GI transit time (22). Opioids also lead to an alteration of intestinal fluid secretion through a direct effect on the enteric nervous system as opioids activate submucosal receptors causing decreased electrolyte and water secretion into the lumen in addition to an increased fluid absorption across the intestinal wall (23,24).
Opioids have been previously shown to inhibit GI motility, causing some researchers to suggest that their use may be associated with a prolonged gastric transit time in patients receiving a CE examination (25). Several studies have attempted to evaluate the association between CE completion and opioid use with contradicting results. With the exponential growth of opioid use in recent years, understanding these medications’ role in GI motility is crucial (26,27). Therefore, we performed a comprehensive systematic review and meta-analysis to evaluate the association between opioid use and CE completion in published literature.
Methods
Search strategy and selection criteria
We performed a comprehensive literature search in PubMed, PubMed Central, Embase, and ScienceDirect databases from inception through June 1, 2018, to identify all the studies that evaluated the association between CE completion and opioid use. We used the following keywords in different combinations: capsule, endoscopy, opioids, narcotics. The search was limited to human studies with no restrictions placed on region, publication type, or language.
Data extraction and quality assessment
We included studies that evaluated the effects of opioids on CE completion rates only if they presented an odds ratio (OR) for our main outcome with a 95% confidence interval (CI) or presented the data sufficient to calculate the OR with a 95% CI. Studies were excluded for the following reasons: (I) were letters to editors, case reports, case series, and review articles or (II) provided insufficient information to calculate CE completion rates and/or the OR for our main outcome.
The authors independently performed the literature review. The data was extracted and reviewed for accuracy prior to analysis. Risk of internal bias was assessed using the Newcastle-Ottawa scale (28).
Statistical analysis
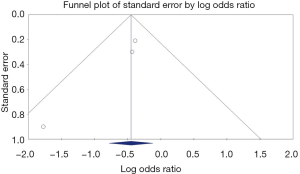
Statistical analysis was performed using the comprehensive meta-analysis (CMA), version 3 software (BioStat, Inc., Eaglewood, NJ, USA). Effect estimates from the individual studies were extracted and combined using the random-effect, generic inverse variance method of DerSimonian and Laird (29). A random-effect model was used as a high probability of between-study variance was suspected due to variation in study population and methodology. A pooled OR was calculated. A Cochran’s Q-test and an I2 statistic were used to evaluate heterogeneity and quantify variation across the selected studies (30). A funnel plot was then created to evaluate for publication and other reporting biases and then the plot was examined visually for asymmetry. Then, an Egger test for asymmetry of a funnel plot was conducted.
Results
Search results
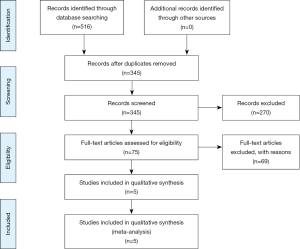
Our search yielded 516 citations. All citations underwent a title and abstract review, resulting in 75 citations which underwent a full-length article review. Sixty-nine were excluded as they did not include controls, were review articles, or did not provide sufficient information to calculate CE completion rates and/or the OR for our main outcome. Of note, Yazici et al. [2012] published a P value in their examination of opioid association with CE completion; however, the authors were contacted and were able to provide us OR or data sufficient to calculate it for the study to be included in this meta-analysis (31). A flow diagram illustrates the selection process, Figure 1. Consequently, a total of five studies met our inclusion criteria and were included in the meta-analysis (32-36).
Meta-analysis results
CE completion rate
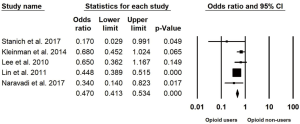
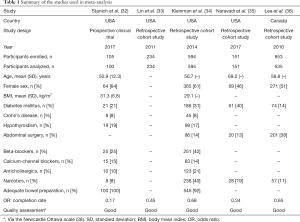
Characteristics of the included studies are summarized in Table 1. Five studies published between the years 2010–2017 with a total of 1,614 patients undergoing CE in the inpatient (IP) and outpatient (OP) setting were included in this study (32-36). Two of these studies were only available in abstract form at the time of writing (33,35). Four studies took place in the United States and one Canada (32-36). The inclusion and exclusion criteria of the involved studies are summarized in Table 2. Of these 1,614 patients, 349 had an incomplete CE (21.6%). The pooled OR for CE completion is 0.50 (95% CI: 0.38–0.66, I2=36.9%) in opioid users compared to non-users, Figure 2.
Full table

Full table
Subgroup analysis
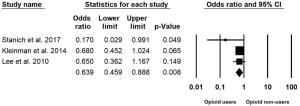
Analysis was performed on three studies after excluding the two studies that were only available in abstract form given the limited data available to account for publication bias. The pooled OR for CE completion remained statistically significant at 0.639 (95% CI: 0.459–0.888, I2=11.5%) in opioid users compared to non-users, Figure 3.
Evaluation for publication bias
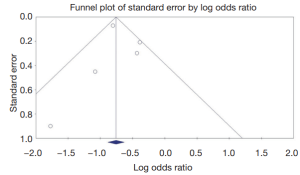
A Funnel plot was generated to evaluate CE completion rates (Figures 4,5). The plot for all studies is symmetric and does not suggest the presence of publication bias. Egger’s regression asymmetry testing was also done to demonstrate no evidence of publication bias (P>0.05).
Discussion
Since being introduced approximately 20 years ago, CE has become an essential diagnostic tool for detecting GI luminal pathologies worldwide. More recently, researchers have focused their efforts toward investigating CE efficacy, limitations, and risk factors for incomplete examination. A considerable but inconsistent body of research has been put forward to evaluate the association between CE completion and opioid use. For this reason, the present meta-analysis aimed to examine the previously published studies on this association. In the current investigation, we found that opioid users are 50% less likely to have a complete CE examination compared to non-users. Our results suggest that opioids should be avoided prior to CE testing to limit capsule-related complications, medical system costs, and prolonged hospital stays.
Our analysis included five studies with a total of 1,614 patients undergoing CE in both the IP and OP setting, 349 of which had an incomplete CE (21.6%). The first paper analyzed, Stanich et al. [2017], is a prospective study of the association between physical activity and CE bowel transit (32). One hundred and five patients in the OP setting were analyzed. Patients with need for a patency capsule, patients requiring endoscopic delivery of the CE, patients with a previous gastric or small-bowel surgery including resection, patients with a diagnosis of gastroparesis and/or use of prokinetics such as metoclopramide or azithromycin, patients with a history of previous CE retained in the stomach for the duration of the battery life, and patients who are wheelchair bound were all excluded.
Their data demonstrate that the use of narcotic medications is associated with incomplete CE (OR: 0.17, P=0.07, 95% CI: 0.03–1.08) but that no significant difference exists in total transit time (OR: 0.73, P=0.46, 95% CI: 0.32–1.68) among opioid users having a CE examination. The study is unique in its prospective design as well as having impressively thorough exclusion criteria, minimizing the risk of bias. However, the study is limited by its small size and by only including patients in the IP setting. Within their study, only seven patients had incomplete CE examinations.
Kleinman et al. [2014] investigated the effect of opioid use on CE in a retrospective study (34). Unlike Stanich et al. [2017], this study reviewed CE examination data from both IP and OP cohorts. Their study compared transit times via log-rank analysis on 594 adults and found that gastric transit time was not significantly different between opioid and non-opioid users in the OP setting. However, their data showed gastric transit time was prolonged in patients receiving opioids in the IP setting and a trend in the number of gastric transit time greater than 45 minutes in the opioid group (300 vs. 260 minutes, P=0.11). Of note, prolonged gastric transit time greater than 45 minutes has been found to be associated with increased rates of incomplete CE examinations (37). Additionally, after multivariate analysis was conducted and adjustments were made for the cohorts, total transit time was significantly longer in patients using opioids (44 vs. 23 minutes, P=0.04).
Despite the study being primarily limited by its retrospective design, Kleinman et al. [2014] compared relatively large cohorts. Unfortunately, as discussed by the authors, their study did not delineate opioid dosing among patients. Without knowing patients’ narcotic dose and duration, the confounding effect is unaccounted for in this study. Moreover, patients in the non-opioid cohort were older and more likely to have other known risk factors for prolonged gastric transit time and incomplete CE examinations, such as diabetes mellitus (38).
Lin et al. [2011] performed a retrospective cohort study that evaluated 234 cases of CE in both the IP and OP setting (33). Univariate analysis demonstrated higher completion rates were associated with patients who had no previous history of narcotic use (OR: 2.23, P=0.039) or had not received previous sedation (OR: 3.648, P=0.001). This assertion by Lin et al. [2011], would seem to explain and support the incongruence in data between the IP and OP setting seen in Kleinman et al. [2014].
Another retrospective cohort study by Naravadi et al. [2017], aimed to determine the factors that impact completion of CE in patients admitted to Loyola University Medical Center in Maywood, Illinois (35). They reviewed medical records of 151 adult patients who had symptomatic iron deficiency anemia or overt OGIB requiring CE examination performed in the IP setting between January 2014 and October 2016. Notably, the study excluded patients with ileostomy, studies with equipment malfunction, repeat CE studies during the same admission, and 12-hour capsule studies (35). The study found a significant difference in CE study completion in patients who had used opiates (P=0.01) and concluded that opiate use is a risk factor for incomplete CE in the IP setting regardless of method of capsule ingestion. Unfortunately, as in Kleinman et al. [2014], the Navaradi et al. [2017] study did not delineate opioid dosing among patients.
Our fifth included study by Lee et al. [2010], is a retrospective study analyzing 535 CE procedures (36). Patients with hemiplegia or diabetes with end organ damage were excluded, and patients with evidence of mass lesions or stricture that was responsible for delaying transit through the small bowel were excluded from the study as well. Their data showed that opiate medication use (P=0.094) was not statistically associated with incomplete CE studies but did show a trend toward incomplete CE examination (OR: 1.54, P=0.15, 95% CI: 0.86–2.76). Again, the study is primarily limited by its retrospective design. Additionally, the authors noted that their study did not control for bowel preparation before CE examination given the fact that the wide variation in preparation might confound the completion rate data.
The studies used in our meta-analysis included patients in both the IP and OP setting. IP status was noted by several authors to inversely correlate with completion rates, likely because IP status is a reflection of decreased mobility and reduced overall health. Furthermore, there may be an increased likelihood of acute narcotic use in the IP setting at varying doses (31,39,40). The factors that significantly correlated with transit time and CE completion rate varied by study. Those factors included: patient age, mobility, history of bowel obstruction, history of major abdominal surgery, overt GI bleeding, bowel obstruction, diabetes, indication for CE, and method of ingestion (32-36). Other studies not included in this analysis have demonstrated multiple independent risk factors for incomplete CE examinations, including previous small intestine surgery, hospitalization, moderate or poor bowel cleansing, and a gastric transit time longer than 45 minutes (37). Researchers have recently theorized that metoclopramide may increase the likelihood of complete small intestine examination because of its prokinetic effects on the GI tract; however, literature review found contradicting data (41,42).
Opioids have been shown to predict inadequate quality colon preparation in a dose-dependent manner (43). For example, methadone dependence was found to be a risk factor for poor bowel visualization during endoscopy and increased number of repeat colonoscopies (44). Additionally, patients on high dose opioids are at a higher risk for a prolonged endoscopic procedure time and increased procedural discomfort (45). The dysmotility action of opioids has been shown to be dose-related and cumulative as well (46). Our meta-analysis adds to this growing body of research, showing that opioid use while undergoing CE can prolong transit time and the likelihood of incomplete imaging, however, none of the studies included in this analysis studied the correlation between opioid doses and CE completion rates.
To our knowledge, our study represents the first meta-analysis to assess the association between CE completion and opioid use. Together, the studies comprising this meta-analysis are a relatively large sample size compared to any previously published data on the topic. From our results, we suggest practitioners weigh the benefits of withholding opioids for hospitalized patients or consider changing OP medication regimens for those undergoing CE.
Our study was unable to evaluate optimal opioid dosing and duration of use with respect to CE completion rates. Currently, multiple government and academic institutions have their own recommendations for withholding opioids prior to CE, though these recommendations vary significantly between institutions (47). Future direction of research may also aim to investigate prokinetic agents, such as metoclopramide and erythromycin which have been theorized to improve completion rates (48).
Our results should be interpreted within the context of our study design and several limitations are present. First, the quality of available primary studies and selection bias were inherent limitations in performing this meta-analysis. Despite our analysis including different studies of variable methodological quality due to limited available literature, our results proved to be reproducible using a rigorous sensitivity analysis. Additionally, our study is limited by the fact that two of the studies included in the analysis were only available in abstract form at the time of writing with incomplete description of their methodology (33,35). Another potential limitation is the possible differences in the PillCam capsule technology used between these studies. For example, there might have been upgrades to the capsule technology between 2001 and 2016 (35,36). Likewise, different bowel preparation methods were used between the studies which may potentially affect CE completion rates. While several limitations exist, we believe our study offers important insight into the effect of opioids on CE completion.
Conclusions
In summary, our results indicate that opioid use is associated with significantly lower rates of CE completion. To our knowledge, this is the first meta-analysis to evaluate this association. Future prospective randomized research is needed to examine the effect of withholding opioids prior to CE on completion rates.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol 2005;100:2407-18. [Crossref] [PubMed]
- Saperas E, Dot J, Videla S, et al. Capsule endoscopy versus computed tomographic or standard angiography for the diagnosis of obscure gastrointestinal bleeding. Am J Gastroenterol 2007;102:731-7. [Crossref] [PubMed]
- Liao Z, Gao R, Xu C, et al. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc 2010;71:280-6. [Crossref] [PubMed]
- Culliford A, Daly J, Diamond B, et al. The value of wireless capsule endoscopy in patients with complicated celiac disease. Gastrointest Endosc 2005;62:55-61. [Crossref] [PubMed]
- Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer 2006;107:22-7. [Crossref] [PubMed]
- Gastineau S, Viala J, Caldari D, et al. Contribution of capsule endoscopy to Peutz-Jeghers syndrome management in children. Dig Liver Dis 2012;44:839-43. [Crossref] [PubMed]
- Ho KK, Joyce AM. Complications of capsule endoscopy. Gastrointest Endosc Clin N Am 2007;17:169-78. viii-ix. [Crossref] [PubMed]
- Rondonotti E, Herrerias JM, Pennazio M, et al. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc 2005;62:712-6; quiz 752, 754.
- Cheifetz AS, Sachar DB, Lewis BS. Small bowel obstruction—indication or contraindication for capsule endoscopy. Gastrointest Endosc 2004;59:102. [Crossref]
- Yang XY, Chen CX, Zhang BL, et al. Diagnostic effect of capsule endoscopy in 31 cases of subacute small bowel obstruction. World J Gastroenterol 2009;15:2401-5. [Crossref] [PubMed]
- Bailey AA, Debinski HS, Appleyard MN, et al. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three-center Australian experience. Am J Gastroenterol 2006;101:2237-43. [Crossref] [PubMed]
- Rondonotti E, Pennazio M, Toth E, et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy 2008;40:488-95. [Crossref] [PubMed]
- Cheon JH, Kim YS, Lee IS, et al. Can we predict spontaneous capsule passage after retention? A nationwide study to evaluate the incidence and clinical outcomes of capsule retention. Endoscopy 2007;39:1046-52. [Crossref] [PubMed]
- Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept 2009;155:11-7. [Crossref] [PubMed]
- Lammers WJ, Lammers-van den Berg AM, Morrison JF, et al. Translating Trendelenburg; back to the future. Naunyn Schmiedebergs Arch Pharmacol 2006;373:134-8. [Crossref] [PubMed]
- Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil 2004;16 Suppl 2:17-28. [Crossref] [PubMed]
- Stacher G, Steinringer H, Schneider C, et al. Effects of the prodrug loperamide oxide, loperamide, and placebo on jejunal motor activity. Dig Dis Sci 1992;37:198-204. [Crossref] [PubMed]
- Pannemans J, Corsetti M. Opioid receptors in the GI tract: targets for treatment of both diarrhea and constipation in functional bowel disorders? Curr Opin Pharmacol 2018;43:53-8. [Crossref] [PubMed]
- Davis MP. The opioid bowel syndrome: a review of pathophysiology and treatment. J Opioid Manag 2005;1:153-61. [Crossref] [PubMed]
- Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract 2007;61:1181-7. [Crossref] [PubMed]
- De Luca A, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther 1996;69:103-15. [Crossref] [PubMed]
- Holzer P. Treatment of opioid-induced gut dysfunction. Expert Opin Investig Drugs 2007;16:181-94. [Crossref] [PubMed]
- Paulson DM, Kennedy DT, Donovick RA, et al. Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction--a 21-day treatment-randomized clinical trial. J Pain 2005;6:184-92. [Crossref] [PubMed]
- Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett 2004;361:192-5. [Crossref] [PubMed]
- Kleinman B, Stanich P, Porter K, et al. Opioid use is associated with prolonged gastric transit time in hospitalized patients receiving video capsule endoscopy. Am J Gastroenterol 2013;108:S495. [Crossref]
- Grunkemeier DM, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007;5:1126-39. [Crossref] [PubMed]
- Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835-42. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute, 2014.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Yazici C, Losurdo J, Brown MD, et al. Inpatient capsule endoscopy leads to frequent incomplete small bowel examinations. World J Gastroenterol 2012;18:5051-7. [Crossref] [PubMed]
- Stanich PP, Peck J, Murphy C, et al. Physical activity during video capsule endoscopy correlates with shorter bowel transit time. Endosc Int Open 2017;5:E856-60. [Crossref] [PubMed]
- Lin J, Cohen BL, Savides TJ, et al. Inpatient status is associated with decreased rates of capsule endoscopies reaching the cecum. Gastroenterology 2011;140:S-768. [Crossref]
- Kleinman B, Stanich PP, Betkerur K, et al. Opioid use is not associated with incomplete wireless capsule endoscopy for inpatient or outpatient procedures. Diagn Ther Endosc 2014;2014:651259. [Crossref] [PubMed]
- Naravadi VVR, Balasubramanian N, Wanta K, et al. Su1176 predictors of incomplete capsule endoscopy in hospitalized patients. Gastrointest Endosc 2017;85:AB302. [Crossref]
- Lee MM, Jacques A, Lam E, et al. Factors associated with incomplete small bowel capsule endoscopy studies. World J Gastroenterol 2010;16:5329-33. [Crossref] [PubMed]
- Cave DR, Fleischer DE, Leighton JA, et al. A multicenter randomized comparison of the Endocapsule and the Pillcam SB. Gastrointest Endosc 2008;68:487-94. [Crossref] [PubMed]
- Triantafyllou K, Kalantzis C, Papadopoulos AA, et al. Video-capsule endoscopy gastric and small bowel transit time and completeness of the examination in patients with diabetes mellitus. Dig Liver Dis 2007;39:575-80. [Crossref] [PubMed]
- Westerhof J, Weersma RK, Koornstra JJ. Risk factors for incomplete small-bowel capsule endoscopy. Gastrointest Endosc 2009;69:74-80. [Crossref] [PubMed]
- Robinson CA, Jackson C, Condon D, et al. Impact of inpatient status and gender on small-bowel capsule endoscopy findings. Gastrointest Endosc 2011;74:1061-6. [Crossref] [PubMed]
- Selby W. Complete small-bowel transit in patients undergoing capsule endoscopy: determining factors and improvement with metoclopramide. Gastrointest Endosc 2005;61:80-5. [Crossref] [PubMed]
- Almeida N, Figueiredo P, Freire P, et al. The effect of metoclopramide in capsule enteroscopy. Dig Dis Sci 2010;55:153-7. [Crossref] [PubMed]
- Kushnir VM, Bhat P, Chokshi RV, et al. The impact of opiate pain medications and psychoactive drugs on the quality of colon preparation in outpatient colonoscopy. Dig Liver Dis 2014;46:56-61. [Crossref] [PubMed]
- Verma S, Fogel J, Beyda DJ, et al. Chronic methadone use, poor bowel visualization and failed colonoscopy: a preliminary study. World J Gastroenterol. 2012;18:4350-6. [Crossref] [PubMed]
- Nusrat S, Mahmood S, Bitar H, et al. The impact of chronic opioid use on colonoscopy outcomes. Dig Dis Sci 2015;60:1016-23. [Crossref] [PubMed]
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008;11:S105-20. [PubMed]
- Koulaouzidis A, Douglas S. Capsule endoscopy in clinical practice: concise up-to-date overview. Clin Exp Gastroenterol 2009;2:111-6. [Crossref] [PubMed]
- Ponferrada A, Gonzalez-Asanza C, Menchen L, et al. Predictive factors of incomplete capsule endoscopy examination. Gastrointest Endosc 2005;61:AB178. [Crossref]
Cite this article as: Al Momani L, Alomari M, Bratton H, Boonpherg B, Aasen T, El Kurdi B, Young M. Opioid use is associated with incomplete capsule endoscopy examinations: a systematic review and meta-analysis. Transl Gastroenterol Hepatol 2020;5:5.