
Cholecystectomy does not worsen progression or outcomes in non-alcoholic fatty liver disease
Introduction
The unrelenting increase in obesity in the general American population has increased the risk for development of metabolic syndrome (MS), which is defined by Adult Treatment Panel III (ATP III) as abdominal obesity, dyslipidemia, hypertension and hyperglycemia. It is estimated that currently approximately 22% of the US adults have MS (1). Gall stone disease and non-alcoholic fatty liver disease (NAFLD) share common risk factors of hypertriglyceridemia, abdominal obesity and diabetes (2), with 63% increased odds for gallbladder disease with every 5 units increase in body mass index (BMI) (3). Hence, NAFLD and cholelithiasis are frequently seen to co-exist in clinical practice, and it is estimated that 55% of patients with gallstones also carry a diagnosis of non-alcoholic steatohepatitis (NASH) (4,5). Moreover, cirrhosis is also a recognized risk factor for development of cholesterol gallstones (2).
Cholecystectomy is one of the most common procedures performed in the US for symptomatic gall bladder disease (6). Data remains unclear on whether increases risk for development of NAFLD. Cholecystectomy has recently been shown to be an independent risk factor for development of NAFLD (7). Speculated mechanisms are alteration in bile acid circulation from absence of gall bladder (8), altered triglyceride metabolism (9), and loss of metabolic activity of gallbladder notably through decreased secretions of fibroblast growth factor (FGF 19) (10). This retrospective study was performed to examine the question whether cholecystectomy increases risk for and progression of NAFLD development or is just an association in the natural course of NAFLD patients. To examine this hypothesis, we assessed the outcomes of patients with NAFLD depending upon the timing of cholecystectomy whether before or after the diagnosis of NAFLD.
Methods
Study population
Five hundred eighty-four patients steatohepatitis related liver disease [205 from alcoholic liver disease (ALD) and 379 due to NAFLD] seen and managed at a tertiary medical center during 2004 to 2011 were retrospectively analyzed. Of these 191 had undergone cholecystectomy.
Definitions
Non-ALD
Diagnosed by documentation of hepatic steatosis and exclusion of other causes of liver disease including harmful alcohol use of >30 g/day in females and >50 g/day in males (11).
ALD
Diagnosed by consumption of alcohol of >30 g/day in women and >50 g/day in men for >5 years and exclusion of other causes of liver disease (12).
NAFLD activity score and fibrosis stage
Liver biopsies on patients receiving this procedure were evaluated for steatosis grade (score 0–3), lobular inflammation severity (score 0–2), and ballooning (score 0–2). The total score from these three components ≥5 was consistent with diagnosis of NASH (13). Fibrosis was evaluated on histology and staged for F0 to F4 as per Metavir criteria (14).
Cholecystectomy
Defined by history of and/or absence of gallbladder on ultrasonography.
Cirrhosis
Diagnosed by clinical, hematological, and imaging criteria and/or biopsy when available (15). Patients diagnosed with cirrhosis at or within six months of their presentation were excluded in order to consider for time gap in coding for cirrhosis diagnosis.
Complications of liver disease
Complications of liver disease including ascites, encephalopathy, variceal bleeding, and HCC were defined using available clinic documentation including clinical notes, endoscopic reports and radiological imaging reports. Hepatocellular carcinoma (HCC) was diagnosed based on the American Association for the Study of Liver Diseases (AASLD) guidelines and criteria using computed tomography (CT)/magnetic resonance imaging (MRI) scan and/or biopsy when performed (16). Patients diagnosed with cirrhosis at or within one month of their presentation were excluded to consider for time gap in coding for the respective complication.
Patient survival
Status was recorded from the chart review and confirm with National Death Index using the social security number.
Data extraction
Electronic medical records of patients identified with ALD or NAFLD using the ICD-09 billing codes were analyzed for stratifying the diagnosis (ALD or NAFLD), date of diagnosis, patient demographics (age, gender, race, BMI), comorbidities (diabetes mellitus, dyslipidemia, hypertension), history of cholecystectomy and its date, cirrhosis, complications of liver disease (ascites, variceal bleeding, hepatic encephalopathy, or HCC), laboratory values, histology findings on patients receiving this procedure, last follow up date, date of death if patient died, and last follow up date. Disease progression was assessed.
Data analyses
Baseline characteristics comparing patients with and without history of cholecystectomy were compared for the entire group and separately for patients with NAFLD. Logistic regression analyses models were built to determine factors associated with cholecystectomy for the entire sample and separately for NAFLD group. Patients with NAFLD and cholecystectomy were stratified based on timing of cholecystectomy to group 1 (before the NAFLD diagnosis) and group 2 (at or after the NAFLD diagnosis). The two groups were compared for disease progression determined by time to development of cirrhosis, complications of liver disease, and patient mortality at 5 years from the date of NAFLD diagnosis. For this analysis, patients lost to follow up and those without event during this period were censored.
Statistical analysis
Baseline characteristics were compared using chi-square and t-tests for categorical and continuous variables respectively. Odds ratio (OR) and 95% confidence interval (CI) were used to report analyses on logistic regression model analyses. Kaplan-Meier curves were derived comparing 5-year outcomes on NAFLD patients comparing the two groups based on timing of cholecystectomy, with log-rank test was statistical test used. All statistical comparisons were performed using Statistical Analysis Software (SAS Inc., Cary, NC, USA) and P value <0.05 were considered significant.
Results
Baseline characteristics of patients with steatohepatitis related liver disease
Of 584 patients, 191 with compared to 393 without cholecystectomy were more likely to be females, have features of MS, NAFLD diagnosis, and cirrhosis at presentation (Table 1). On a logistic regression model controlled for variables different at baseline and clinically relevant variables, NAFLD diagnosis was strongest factor associated with over 3 folds odds of having history of cholecystectomy. Other predictors were female gender and cirrhosis at presentation (Table 2).
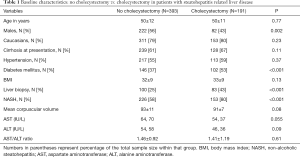
Full table

Full table
Baseline characteristics of patients with NAFLD
Within the subgroup of 379 NAFLD patients, 153 with compared to 226 without cholecystectomy were more likely to be females, liver biopsy for diagnosis, cirrhosis at presentation, and higher aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio (Table 3). On a logistic regression model controlled for variables different at baseline and clinically relevant variables, cirrhosis at presentation and female gender were associated with over 2 and 1.5 folds odds of having history of cholecystectomy respectively (Table 4).
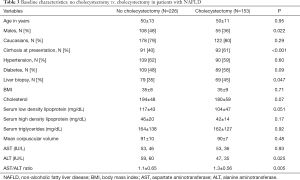
Full table

Full table
Baseline characteristics: cholecystectomy before vs. at or after NAFLD diagnosis
Of 141 NAFLD patients with available information on timing of cholecystectomy, 86 (group 1) patients had cholecystectomy before and 55 (group 2) at or after diagnosis of NAFLD. Median (Interquartile range) time in years between the cholecystectomy and NAFLD diagnosis was 6.2 (1.8–18.4) and 0 (0–1.7) years in groups 1 and 2 respectively (Figure 1). Baseline characteristics comparing the two groups were similar except for higher model for end-stage liver disease (MELD) score in group 2 as compared to group 1 (Table 5).
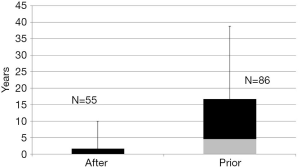
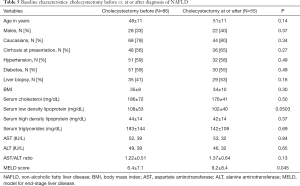
Full table
Liver histology: cholecystectomy before vs. at or after NAFLD diagnosis
Of 141 patients with available information on cholecystectomy, liver histology data was available on 48 patients, with 28 group 1 (cholecystectomy before NAFLD diagnosis). The mean NAFLD activity score was higher in group 1 compared to group 2 without differences on the different components of this score (Table 6). The fibrosis stages were also similar comparing the two groups with 10–20% and 25–36% of patients at extremes without any fibrosis and with cirrhosis respectively in the two groups (Table 6).

Full table
Outcomes: cholecystectomy before vs. at/after NAFLD diagnosis (groups 1 vs. 2)
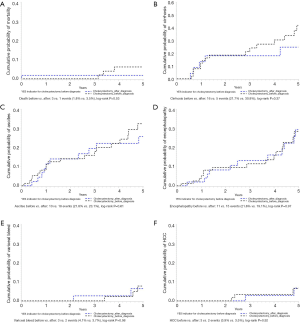
Five-year patient outcomes among NAFLD patients were compared for group 1 vs. 2 based on timing of cholecystectomy. Among 141 patients followed for 5 years, 4 patients died (3, group 1), with cumulative probability of patient mortality of 1.8% vs. 3.5%, P=0.53 (Figure 2A). Excluding 66 patients (52, group 1) with diagnosis of cirrhosis in the first 6 months from the time of NAFLD diagnosis, 21 patients (16, group 1) developed cirrhosis over 5-year with cumulative probability of 21.7% vs. 30.8%, P=0.37 (Figure 2B). Similarly, after excluding patients with respective complication of cirrhosis in the first month from diagnosis, ascites was the most common followed by hepatic encephalopathy, variceal bleeding, and HCC. Among 124 patients (78, group 1), ascites developed in 28 patients (10, group 1) over 5-year period with cumulative probability of 21.8% vs. 23.1%, P=0.61 (Figure 2C). Among 134 patients (83, group 1), hepatic encephalopathy developed in 26 patients (11, group 1) over 5-year period with cumulative probability of 21.6% vs. 19.1%, P=0.97 (Figure 2D). Among 131 patients (82, group 1), variceal bleed developed in 5 patients (3, group 1) over 5-year period with cumulative probability of 4.1% vs. 3.7%, P=0.98 (Figure 2E). Among 136 patients (85, group 1), HCC developed in 5 patients (3, group 1) over 5-year period with cumulative probability of 3.9% vs. 3.5%, P=0.92 (Figure 2F).
Discussion
The results of our retrospective analysis on patients with steatohepatitis related liver disease (ALD and NAFLD) show that NAFLD diagnosis is associated with history of cholecystectomy. Further, among patients with diagnosis of NAFLD, cholecystectomy timing (before vs. at or after the NAFLD diagnosis) is not associated with severity and progression of the disease. This suggests that increased prevalence of cholecystectomy among NAFLD patients is an association due to sharing the common risk factors and does not have a causative relationship with the development or progression of NAFLD.
With the increase in prevalence of obesity and MS in the US, prevalence of NAFLD, a hepatic manifestation of MS is increasing and is emerging as an epidemic with one of the commonest causes of liver disease, cirrhosis with its complications, and requirement of liver transplant (4). The mechanism of progression of NAFLD to more sinister and advanced spectrum is speculated to be centered around ‘two-hit’ hypothesis. The ‘first hit’ is marked by increased levels of triglyceride (TG) in the hepatocytes with lipotoxicity and the second hit from multiple sources including upregulation of inflammation with pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-1β, oxidative stress, changes in gut microbiome, and activation of hepatic stellate cells with laying down of collagen (17). Other factors which have been implicated in progression of NASH which are currently being studies are genetics (18), chromosomal abnormalities and environmental factors (19,20).
One area that has gained significant amounts of interest lately is the role of bile acids and the farnesoid X receptor (FXR) in the pathogenesis of NASH. FXR plays a large role in controlling gluconeogenesis and glycogenolysis in the liver (21), insulin secretion by the pancreas (22), increased production of FGF 19 (23), with improvement in dyslipidemia, hepatic steatosis and insulin sensitivity (24). Treatment of patients with obeticholic acid, as synthetic derivative of the primary bile acid chenodeoxycholic acid, improved insulin sensitivity and reduced markers of liver fibrosis and inflammation (25). While the gallbladder is considered the main extra hepatobiliary source of FGF 19, the liver is able to compensate in its absence to maintain homeostasis (26).
Cholecystectomy alters bile acid physiology, metabolism and circulation. While there appears to be initial increase after surgery, bile acids reach a steady state at three months after cholecystectomy, with amount and turnover unchanged compared to non-surgical patients (8). It has been shown that there is no change in bile acid pool and its composition in the long-term after cholecystectomy (27). The data from these studies are consistent with results on our retrospective analysis in this study.
However, results of our study are contrary to a recently published study showing cholecystectomy to be an independent risk factor for NAFLD (7). In this study based on analysis of National Health and Nutrition Examination Survey (NHANES) data (1988–1994) on over 12,000 participants, prevalence of NAFLD was 20% in the entire cohort (48% among patients with cholecystectomy, 34% among those with gall stones, and 18% among patients without gall stone disease, P<0.01 for all comparisons). Cholecystectomy was associated with diagnosis of NAFLD with over two folds odds as in our study. Based on these data, the authors concluded that cholecystectomy may itself be a risk factor for NAFLD (7). However, the study did not examine any temporal association of cholecystectomy with the NAFLD diagnosis as we performed in this study. Further, it is known that cholecystectomy is often performed for indications other than gall stones and these patients share the common risk factors as NAFLD patients.
Improved methodology compared to earlier study and well defined cohort of steatohepatitis related liver disease with medical chart review is a strength of our study. However, our study apart from limitations of retrospective study, is limited by data from a single center without internal or external validation and lack of data availability on timing of cholecystectomy in about 25% of patients with history of cholecystectomy.
In summary, cholecystectomy has strong association with diagnosis of NAFLD, but was not associated with the risk of development or progression to more advanced spectrum of NAFLD. Future prospective studies are needed to validate these findings.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Institutional IRB (X130619002).
References
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9. [Crossref] [PubMed]
- Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol 2006;20:981-96. [Crossref] [PubMed]
- Aune D, Norat T, Vatten LJ. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol 2015;30:1009-19. [Crossref] [PubMed]
- Yener O, Aksoy F, Demır M, et al. Gallstones associated with nonalcoholic steatohepatitis (NASH) and metabolic syndrome. Turk J Gastroenterol 2010;21:411-5. [Crossref] [PubMed]
- García-Monzón C, Vargas-Castrillón J, Porrero JL, et al. Prevalence and risk factors for biopsy-proven non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in a prospective cohort of adult patients with gallstones. Liver Int 2015;35:1983-91. [Crossref] [PubMed]
- National Center for Health Statistics. Health, United States, 2009: with special feature on medical technology. Hyattsville: Centers for Disease Control and Prevention, 2010.
- Ruhl CE, Everhart JE. Relationship of non-alcoholic fatty liver disease with cholecystectomy in the US population. Am J Gastroenterol 2013;108:952-8. [Crossref] [PubMed]
- Berr F, Stellaard F, Pratschke E, et al. Effects of cholecystectomy on the kinetics of primary and secondary bile acids. J Clin Invest 1989;83:1541-50. [Crossref] [PubMed]
- Amigo L, Husche C, Zanlungo S, et al. Cholecystectomy increases hepatic triglyceride content and very-low-density lipoproteins production in mice. Liver Int 2011;31:52-64. [Crossref] [PubMed]
- Barrera F, Azócar L, Molina H, et al. Effect of cholecystectomy on bile acid synthesis and circulating levels of fibroblast growth factor 19. Ann Hepatol 2015;14:710-21. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57. [Crossref] [PubMed]
- Singal AK, Bataller R, Ahn J, et al. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol 2018;113:175-94. [Crossref] [PubMed]
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21. [Crossref] [PubMed]
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994;20:15-20. [Crossref] [PubMed]
- Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology 2009;49:1017-44. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Onyekwere CA, Ogbera AO, Samaila AA, et al. Nonalcoholic fatty liver disease: synopsis of current developments. Niger J Clin Pract 2015;18:703-12. [Crossref] [PubMed]
- Browning JD, Kumar KS, Saboorian MH, et al. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol 2004;99:292-8. [Crossref] [PubMed]
- Bochukova EG, Huang N, Keogh J, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature 2010;463:666-70. [Crossref] [PubMed]
- Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology 2008;48:1791-8. [Crossref] [PubMed]
- Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 2006;116:1102-9. [Crossref] [PubMed]
- Düfer M, Hörth K, Wagner R, et al. Bile acids acutely stimulate insulin secretion of mouse β-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes 2012;61:1479-89. [Crossref] [PubMed]
- Modica S, Petruzzelli M, Bellafante E, et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 2012;142:355-65.e1-4.
- Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004;145:2594-603. [Crossref] [PubMed]
- Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013;145:574-82.e1. [Crossref] [PubMed]
- Zweers SJ, Booij KA, Komuta M, et al. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology 2012;55:575-83. [Crossref] [PubMed]
- Kullak-Ublick GA, Paumgartner G, Berr F. Long-term effects of cholecystectomy on bile acid metabolism. Hepatology 1995;21:41-5. [Crossref] [PubMed]
Cite this article as: Kakati D, Kumar U, Russ K, Shoreibah M, Kuo YF, Jackson B, Singal AK. Cholecystectomy does not worsen progression or outcomes in non-alcoholic fatty liver disease. Transl Gastroenterol Hepatol 2020;5:3.


