
Peri-transplant management of nonalcoholic fatty liver disease in liver transplant candidates
Nonalcoholic steatohepatitis (NASH) is currently the second leading cause for liver transplantation (LT) waitlist registration/liver transplantation overall, and in females, the leading cause. It is projected that NASH will likely rise to become the leading indication for LT in males as well (1). According to the United Network for Organ Sharing and Organ Procurement and Transplantation Network data, there was a 162% increase in LT secondary to NASH from 2003 to 2014 (2). NASH cirrhosis is the most rapidly growing indication for ACLF-related hospitalization and use of hospital resources (3). ACLF increased by 24% between 2006 and 2014 with a 63% increase in nonalcoholic steatohepatitis (NASH) cirrhosis (3.5% to 5.7%); a 28% increase in patients with alcoholic cirrhosis (5.6% to 7.2%); a 25% increase in patients with other etiologies (5.2% to 6.5%); and no significant change in patients with viral hepatitis (4.0% to 4.1%) (3). NASH as a cause of LT related to hepatocellular carcinoma (HCC), increased from 8.3% in 2002 to 10.3% in 2007 to 13.5% in 2012. The number of patients undergoing LT for HCC secondary to NASH increased by nearly 4-fold from 2002–2012 (4). NASH patients requiring LT were older and waitlist mortality was higher compared to patients with other etiologies of chronic liver disease (5). Dulai et al. did a systematic review and meta-analysis of 5 studies. Cumulative incidence of death within 3 years of listing for LT was 29% in NASH (6). Factors such as poor performance status, encephalopathy, diabetes, high MELD score, Hispanic race, older age, and a low serum albumin were the main causes of death in patients with NASH who were on the waitlist for LT (7).
NASH is considered as the hepatic manifestation of metabolic syndrome, and as such, the constellation of comorbidities such as diabetes, hypertension, dyslipidemia, and obesity are significantly common in these patients (8). In addition, complications related to these comorbidities such as chronic kidney disease (CKD) and coronary artery disease (CAD) are quite common in NASH patients, and their increased prevalence puts significant clinical challenges in the management of NASH patients on the LT wait-list and during the peritransplant period (9). In this review, we have described these complex challenges in the management of NASH patients with end stage liver disease and attempted to guide clinicians to best manage and prevent future complications with early interventions.
Risk factors affecting graft and patient survival in NASH
Recent meta-analysis of 9 studies showed survival of patients at 1, 3, and 5 years after liver transplantation was similar to other chronic liver disease. Studies that have compared mortality following LT in patients with NASH to post-LT patients with Non-NASH cirrhosis (10-19) are summarized in Table 1. There are unique challenges faced by patients with NASH undergoing LT, a summary of those as well as guideline-based management in the peri-transplant period are summarized in Table 2 (20-38). Patients with NASH are more likely to die from cardiovascular complications or sepsis (39). While some studies showed NASH did not affect graft survival (19), other studies have shown a negative impact of NASH on graft survival, primarily due to underlying metabolic factors (15). Factors including age >60 years, BMI ≥30 kg/m2, pretransplant HTN, and T2DM, have led to increased 30-day and 1-year mortality (18). Both obese patients with BMI more than 40 and underweight patients with BMI less than 18 are associated with increased risk of infectious complications and death (40). Beckman et al. did a meta-analysis of 37 studies and proved the negative effect of obesity on LT outcomes. Patients with BMI >30 had worse patient survival (72.6% and 69.8%) and graft survival (75.8% and 85.4%) than those with normal weight (41). Obesity and type 2 diabetes concomitantly increased 30-day postoperative event rate, length of hospital stay and decreased graft survival (42). Usually post-transplant diabetes can develop within 6–12 months after surgery and these patients have increased rejection and worse survival (43). Close management of the components of metabolic syndrome is crucial to long-term survival and may combat the adverse effects of immunosuppression, improving graft survival and decreasing rates of sepsis. Patients with NASH are known to have poor performance status, which has been linked to decreased graft survival and overall patient 5-year survival rates when compared with the other groups after adjusting for demographic and disease complication factors (44). African American donors are shown to have an increased risk of liver graft loss by 21.5%. When both donor and recipient were African American, graft loss increased by 36.6% (45). Optimization of obesity, hypertension, hyperlipidemia, pre-transplant cardiovascular disease, and smoking status are important in decreasing graft loss in NASH patients.
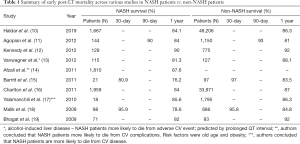
Full table
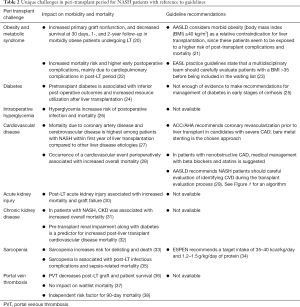
Full table
Donor and allocation issues
Older age, higher BMI, increased prevalence of diabetes and donation after cardiac death (DCD) are leading cause for liver nonuse (46). Miyaaki et al. noted that younger age of recipients and donor steatosis are risk factors for post-LT NASH (47). Zhang et al. conducted meta-analysis of 19 publications to estimate the effect of steatotic livers after LT and noted primary non function rate and early dysfunction rate was higher when moderate and severe steatotic liver donors were used. But graft survival rate and patient survival rate did not differ between steatotic and non steatotic liver donors (27). Recipients receiving liver with macrosteatosis are at increased risk of post reperfusion syndrome, renal dysfunction requiring continuous renal replacement therapy (RRT) following LT, and cardiac arrest compared to donors without steatosis (48). Steatotic grafts with >60% fat are generally not transplanted, while those with 30–60% fat when transplanted have been associated with poor results and should be considered as donors in the absence of other risk factors (49). However, Wong et al. assessed patients who received severely steatotic liver donors and proved even severely steatotic liver donors from low risk donors can be safely used (50). Non-enhanced computed tomography and contrast-enhanced CT attenuation measurements of liver is useful in evaluating steatosis in donor candidates with moderate to severe steatosis (51). Magnetic resonance proton density fat fraction (MR-PDFF) has good negative predictive value for diagnosing donor hepatic steatosis >10% in living donor LTs (52). Zheng et al. did meta-analysis of 8 studies and noted MR imaging and MR spectroscopy has high sensitivity and high specificity for diagnosing hepatic steatosis >10% to >30% in living liver donors (53).
Preoperative and selective intraoperative liver biopsies are proven to be specific compared to imaging studies for assessing donor steatosis and can be considered in patients with abnormal imaging studies to evaluate the liver steatosis on donors (54). Pharmacological enhancement of intracellular lipid metabolism and defatting done during normothermic machine perfusion decreased steatosis in donor livers and reduced the inflammatory cytokines in the perfusate (55). Strategies such as shortened ischemia time, ischemic and pharmacological preconditioning of liver grafts, and the use of machine-based liver perfusion systems are used to optimize fatty liver grafts, which is necessary for deceased liver donors. In patients undergoing living donor LT, Bezafibrate (400 mg/day) for 2–8 weeks in the donors have reduced risk of liver injury in live steatotic grafts (31).
Factors affecting peritransplant outcomes in NASH patients
Obesity and metabolic syndrome
Obesity increases the risk of clinical decompensation in cirrhosis, possibly by increasing portal pressure. Sixteen weeks of diet and moderate exercise were safe and reduced body weight and portal pressure in overweight and obese patients with cirrhosis and portal hypertension (56). The impact of bariatric surgery on LT candidates was assessed by a few studies. Idriss et al. studied 78 adults who underwent liver transplant evaluation after bariatric surgery and noticed that when compared with controls without a history of bariatric surgery, patients with a history of bariatric surgery were more likely to be listed for LT, but a higher rate of delisting or death on the waiting list was noticed in patients with bariatric surgery secondary to malnutrition (57). Sleeve gastrectomy is shown to be a possibly safe alternative that can reduce the metabolic complications in the peritransplant period before and after LT while also decreasing the risk of malnutrition during LT and eliminating the risk of malabsorption of immunosuppressive drugs. Furthermore, sleeve gastrectomy allows for good endoscopic evaluation of varices and biliary complications (58,59).
Patients with morbid obesity had an increased length of stay in the hospital and appeared sick, which required extensive use of hospital resources (60). Obese patients are known to have an increase in mortality while on the waitlist and had decreased post-LT survival. A summary of studies comparing mortality in obese post-LT patients to non-obese post-LT patients (20,61-70,71-77) is summarized in Table 3. Obese patients were less likely to get LT compared to nonobese patients because of excessive post-operative risks (78). With respect to operative outcomes, patients with Class II obesity (BMI >35) or higher MELD scores transplanted for NASH had no difference in operative time, intensive care unit or hospital length of stay, or perioperative complications when compared to non-obese patients undergoing LT (63).
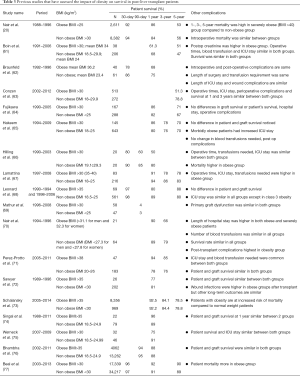
Full table
Studies examining survival outcomes in obese patients undergoing LT have shown conflicting results (79,80). Nair et al. (20) reviewed the UNOS database from 1988 through 1996 and reported increased primary graft nonfunction as well as decreased survival at 30 days, 1-, and 2-year follow-up in morbidly obese patients undergoing LT. Despite these earlier reports, Pelletier et al. (79) demonstrated that there was a survival benefit from transplantation not only for obese patients but also for patients at the extremes of BMI. A recent meta-analysis of 24 studies on 132,162 patients also reported increased mortality risk and higher early postoperative complications, mainly due to cardiopulmonary complications in obese patients after LT compared to the controls (22). Currently, the American Association for the Study of Liver Disease (AASLD), in accordance with the American Society of Transplantation, considers morbid obesity [body mass index (BMI) ≥40 kg/m2] as a relative contraindication for LT, since these patients seem to be exposed to a higher risk of post‐transplant complications and mortality (21). The European Association for the Study of the Liver (EASL) also highlights increased postoperative infections and increased hospital and/or intensive care unit length of stay in obese patients and the EASL practice guidelines state that a multidisciplinary team should carefully evaluate patients with a BMI >35 before being included in the waiting list (23).
A 2013 study that analyzed effectiveness of pre-transplant weight loss in obese patients requiring LT showed that 60% of the cohort gained weight to a BMI greater than 35 kg/m2 post-transplantation (81). Pre-transplant obesity is a strong risk factor for developing post-transplant metabolic syndrome. Idowu et al. stated accumulation of atherogenic lipoproteins caused increased risk of de novo hepatic steatosis after liver transplant (82). Kim et al. noticed about 27.1% had NAFLD and 28.9% had severe steatosis. Obesity at biopsy and preexisting donor graft steatosis are important risk factors for recurrence of NASH after liver transplant (83). Specifically, patients with a BMI greater than 30 kg/m2 are at greatest risk for developing post-transplant metabolic syndrome. A 2005 study by Richards et al. shows that the greatest weight gain occurs after the first 6 months following liver transplant; dietary control at this point is recommended to minimize long-term morbidity and mortality resulting from obesity (84).
Diabetes mellitus
Prevalence of NAFLD is higher in patients with diabetes (85) and is also an independent risk factor for developing diabetes (86,87). Patients with diabetes and NAFLD had a higher rate of hypertension, cardiovascular disease, peripheral arterial disease, hyperlipidemia and cerebrovascular disease, and advanced fibrosis and also increased all-cause mortality, mortality related to cardiovascular disease, and liver disease related mortality (88). A recent study has also concluded that diabetes is associated with an increased risk of HCC in patients with NASH cirrhosis (89). A large national study has reported that pretransplant diabetes is associated with inferior post-operative outcomes and increased resource utilization after LT (24). Pre-transplant diabetes increased risk of portal venous thrombosis which is an independent risk factor of 90-day post-transplant mortality (38).
Management of diabetes in a cirrhotic patient awaiting LT is not without challenge. Diabetes is known to be an independent risk factor for death in liver transplant candidates (90). In cirrhotic patients, fasting glucose may be normal in up to 23% of diabetes cases, and glycated hemoglobin provides falsely low results, especially in advanced cirrhosis (91,92). Similarly, the performance of alternative glucose monitoring tests, such as fructosamine, glycosylated albumin and 1,5-anhydroglucitol, also appears to be suboptimal in chronic liver disease (91). There has been a recent trend for management of these patients by specialists (93).
In a study including 12,442 patients who underwent LT at 63 centers from 2007–2011, pretransplant diabetes was associated with inferior post-operative outcomes and increased resource utilization after LT (24). Additionally, diabetes increases the risk of developing recurrent NASH after LT (94). Machine learning techniques have identified diabetes among other important factors such as recipient age, MELD score, BMI, and dialysis before LT as the strongest predictors for 90-day postoperative mortality (95). Type 2 diabetes, hyperlipidemia, obesity, hypertension, insulin use seems to be important risk factors for the development of recurrent and de novo NAFLD (96,97). Finkenstedt et al. studied 237 transplant recipients and in 255 organ donors and noted that liver transplant recipients with certain genetic characteristics like patatin-like phospholipase domain-containing protein 3 (PNPLA3) is associated with an increased hepatic triglyceride accumulation and recurrence of NASH (98).
The main risk factor for post-LT diabetes is the use of immunosuppressive agents particularly the calcineurin inhibitor (CNI) family (tacrolimus and cyclosporine) (99). New-onset diabetes after transplant (NODAT) adversely affects long-term survival after LT in a manner similar to preexisting diabetes, indicating the need for more aggressive care and closer follow-up, and possibly early post-operative intervention. Sirolimus-based immunosuppression is associated with a significantly higher risk of NODAT than other immunosuppressants (100). Patients with NODAT had reduced survival and an increased incidence of sepsis and chronic renal insufficiency (101). Lastly, steroid free regimens are known to decrease diabetes, hyperlipidemia, cytomegalovirus infections but no difference in patient and graft survival, renal insufficiency, hypertension, neurological disorders and infectious complications were noted (102).
The importance of perioperative glucose control early after LT must be emphasized as the association between the immediate post-transplant glycemic control and the development of subsequent rejection has been well documented (103). Earlier studies have documented that intraoperative hyperglycemia during LT was associated with an increased risk of postoperative infection and mortality (26). Management of blood glucose in the immediate postoperative period with a transition from an insulin drip to a long acting basal insulin along with prandial, rapid-acting insulin for both diabetic and non-diabetic patients was shown to significantly decrease infections up to 1 year from operation when compared to standard glycemic control (104). Aside from these well documented complications acute kidney injury (AKI) (105) and new onset diabetes after transplantation (NODAT) (106,107) have been associated with post-LT variability in glucose control. These studies highlight the importance of post-LT glycemic control to potentially prevent graft failure and complications such as infections. In addition, early peak NODAT has been reported in donor grafts received after circulatory death (DCD) recipients (within 15 days post-LT) (108). A recent meta-analysis has concluded that hyperglycemia in the perioperative period is associated with poor post-LT outcomes (109). With the rising NAFLD population worldwide the need for close monitoring of glucose levels post-LT has become even more important as more patients with diabetes being transplanted. Additionally, these changes have resulted in more donor grafts from older patients with DM and obesity which could be more susceptible to poor outcomes from hyperglycemic stressors (110).
Patients in the immediate perioperative period after liver transplant are in hypercatabolic state where there is increased tissue breakdown but not in hyper metabolic state (111). Patients who has tendency to do uncontrolled eating and emotional eating are at increased risk of worse weight gain >14 kg immediately after liver transplant (112). Post-LT patients secondary to NASH have lower resting energy expenditure and exercise energy expenditure so they will need aggressive diet and exercise regimens to decrease risk of weight gain (113).
So patients are advised increased protein intake 1.3–2 g/kg body weight/day and maintain optimal energy 25–40 kcal/kg/day. Need to continue intake of carbohydrate—50–70% of daily calories with decreased simple sugars and lipids 10–20% of daily calories with increased MUFAs and PUFAs (114). Neto et al. retrospectively reviewed patients about 5 years post liver transplant who followed with multidisciplinary team including nutritionist, endocrinologist working together with surgical team after liver transplant. By adequate control of BP, hyperlipidemia and hyperglycemia, there was an improvement in HbA1c status and weight gain in this study (115). Management of diabetes in liver transplant recipients is not very different compared to pre-transplant diabetes.
Only a few prospective studies have designed interventions aimed at managing post-LT hyperglycemia, post-transplant diabetes mellitus (PTDM) and their impact on post-LT outcomes, and as such, future studies need to be designed to address these issues.
Cardiovascular disease
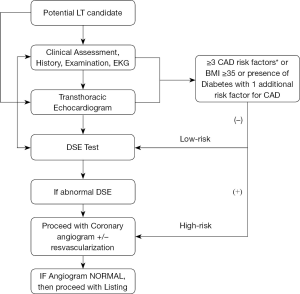
The prevalence of single‐vessel and 3-vessel CAD is significantly higher in patients with NASH cirrhosis compared with HCV and alcoholic cirrhosis (116). Mortality due to CAD and cerebrovascular disease is highest among patients with NASH within first year of LT compared to other liver disease etiologies (117). An algorithm for guiding evaluation for LT in NASH cirrhotic patients from a cardiovascular standpoint is summarized in Figure 1. In general considering their predisposition for CAD a stringent cardiac evaluation is of paramount importance. A transthoracic echocardiogram is required in all patients undergoing liver transplant evaluation to assess the structural and functional capacity of the heart. If patients have more than 2 cardiac risk factors (age >50 years, hypertension, hyperlipidemia, obesity), stress testing should be performed (118). Our center performs stress testing routinely in all patients age >40 years. The two most commonly used non-invasive stress tests are either dobutamine stress echocardiography (DSE) or nuclear perfusion stress testing (SPECT). Patients undergoing DSE should discontinue any beta blocker use 48 hours prior to the procedure as it can cause a false negative result. In our center, the DSE is considered optimal if the LT candidate achieves 85% of maximal heart rate. DSE is quite accurate in diagnosing CAD in general population, but its value in predicting CAD in cirrhotic patients with decompensated has been suboptimal as many patients do not achieve the maximal target heart rate. The sensitivity, specificity, PPV, and NPV in diagnosing obstructive CAD using DSE is 13%, 85%, 22% and 75%, respectively (119). In a recent meta-analysis, the authors found that DSE, myocardial perfusion scintigraphy (MPS), and invasive coronary angiography (ICA) do not satisfactorily predict increased risk of perioperative major adverse cardiac events or all-cause mortality among cirrhotic patients listed for LT, among small and heterogenous studies, questioning the utility of these studies (120). DSE is not recommended in patients with a left bundle-branch block (LBBB) because an increase in heart rate and contractility may cause septal perfusion abnormalities (121). DSE is also contraindicated in patients with atrial fibrillation, atrial flutter, or an automatic implanted cardioverter defibrillator (AICD). In patients with these conditions, nuclear perfusion testing should be performed instead. However, recent studies have shown that noninvasive diagnostic stress tests such as DSE or nuclear perfusion stress test may yield nonspecific results in patients waiting for liver transplant compared to other patients (122). Therefore, in patients with abnormal stress testing, coronary angiography seems to be the gold standard. Additionally, complications from coronary angiography and percutaneous intervention (PCI) were low, making this a safe procedure, per a 2018 study (116). Cardiac catheterization can be safely performed in patients with end stage liver disease despite elevated INR and thrombocytopenia (123). As per ACC/AHA guidelines, coronary revascularization in candidates with severe CAD is frequently performed prior to liver transplant and bare metal stenting was the chosen approach. PCI and revascularization are required in obstructive CAD (greater than 50% reduction in luminal diameter of major coronaries) before a patient can be considered as a potential transplant candidate. In liver transplant candidates requiring bare metal stenting, LT should be delayed by a minimum of 6 weeks (124). In patients with nonobstructive CAD, medical management with beta blockers and statins was suggested.
Intraoperatively, LT results in acute cardiovascular changes, including reduced venous return and sudden increase in peripheral vascular resistance. These are often exacerbated by hemorrhage and reperfusion syndrome, further compromising the already stressed hemodynamics. Patients with end-stage liver disease (ESLD) also have splanchnic and systemic vasodilatation secondary to activation of the renin-angiotensin-aldosterone system. These factors lead to increased flow both in pulmonary and systemic circulations with the resultant elevated pressures in the right ventricle, pulmonary artery, and left atrium in the resting state. Additionally, cirrhotic cardiomyopathy which is noted in 40–50% of cirrhotics, may present with subclinical systolic and diastolic dysfunction, and can be unmasked after LT (125). Therefore, perioperative considerations for cardiovascular disease are significant. As per Vanwagner et al., NASH patients were more likely to have a cardiovascular event within 1 year after LT and about 70% of events occurred in the perioperative period even after controlling for recipient age, sex, smoking status, pretransplant diabetes, cardiovascular disease, and the presence of metabolic syndrome (13).
Predictors for post-transplant cardiovascular disease are age, male sex, diabetes, hypertension, glomerular filtration rate <60 mL/minute, and pre-transplant CVD (126). Minimizing weight gain early after LT can prevent the development of metabolic syndrome and resultant cardiovascular disease (127). Severity or extent of CAD does not impact post-LT survival, if appropriately revascularized (128). Early postoperative cardiac events are associated with inferior survival in liver transplant recipients, irrespective of underlying CAD.
AKI
AKI is a frequent complication after LT. Thongprayoon et al. noted an overall estimated incidence rates of post-LT AKI and severe AKI requiring renal replacement therapy are 40.8% and 7.0%, respectively. There are significant associations of post-LT AKI with increased mortality and graft failure after transplantation (30) In a study including 1,270 patients 34% developed severe AKI, including 18% requiring postoperative RRT. Five factors were identified as the strongest predictors of AKI: donor and recipient BMI, DCD grafts, fresh frozen plasma requirements, and recipient warm ischemia time, leading to a range of 0-25 score points with an AUC (Area under curve) of 0.70. The AKI prediction score is a potential tool to risk stratify recipients at risk for severe post-transplant AKI, and may be of use in early switch to kidney-sparing immunosuppression and early RRT (129). Even in patients with normal preoperative renal function, AKI was a frequent complication in LT recipients and had both negative short- or long-term effects on patient outcomes, also the severity of AKI had a dose-response relationship with worse outcomes. Patients with BMI >25, prolonged inferior vena cava clamping, prolonged cold ischemia time, or post-operative RBC requirement >10 units should be paid particular attention, which may assist in achieving better clinical outcomes (130). NASH as an independent risk factor for renal dysfunction after LT (131). Additionally, recipients with preserved renal function before LT has shown a trend toward lower risk of death with a functioning graft compared with spontaneous liver and kidney transplant (SLKT) recipients and those with pretransplant severe renal dysfunction in patients with NASH. Renal-sparing immunosuppression regimens should be considered at the time of LT to reduce the development of kidney injury in NASH patients.
CKD
Prevalence of CKD ranged from 20% to 55% among patients with NAFLD compared with 5% to 30% among those without NAFLD (132,133). A meta-analysis showed that increased risk of CKD persisted in NASH patients after adjusting for diabetes (134). In patients with diabetic kidney disease, NASH is an independent risk factor for cardiovascular events (135), and in patients with NASH, CKD was associated with increased overall mortality (136). Female sex, pre-transplant CKD, and NASH are independent predictors of development of stage 3 or greater CKD after LT (137). Pre-transplant renal impairment along with diabetes is a predictor for increased post-liver transplant cardiovascular disease mortality (32).
SLKT recipients increased from 6.3% in 2002 to 19.2% in 2011 (138). Patients with preserved renal function before liver transplant were shown to have lower risk of death and increased graft survival compared to those with pre-transplant severe renal dysfunction in patients with NASH (139). Houlihan et al. noted that NASH patients undergoing liver transplant had significantly low EGFR 3 months after LT compared to non-NASH patients even after adjusting for body mass index, tacrolimus levels, diabetes mellitus, hypertension, and HCC (131). Several studies show ACE inhibitors as a treatment for NASH and decreasing the risk of CKD (140-142). ACE inhibitor therapy is thought to be effective in patients with NASH by increasing insulin sensitivity, one of the main pathogenic determinants in NAFLD (134). Pentoxifylline has shown to improve liver tests and also has renal protective action (143,144). Many other drugs like fibrates, thiazolidinediones, epidermal growth factor inhibitors, nuclear factor inhibitors are being studied to improve inflammation and fibrosis related to CKD in NASH patients (145). Their utility in the post-LT period in NASH patients has not been well studied, but appears to be a reasonable strategy.
Sarcopenia and functional status
Sarcopenia is the loss of skeletal mass and associated function and is common in cirrhotic patients due to impaired protein synthesis and inability to adequately store glycogen. Undernutrition, sarcopenia and functional decline increases mortality in waitlist candidate (146). Therefore, management of sarcopenia and frailty is essential in decreasing the dropout rate in waitlist patients. Pretransplant sarcopenia is associated with poor short-term survival post-living donor LT (147). Cirrhotic patients older than 65 years are at particular risk for sarcopenia (148,149). Sarcopenia and overall functional decline in LT candidates on the waitlist has also been shown to be associated with a higher risk of delisting or mortality despite a low baseline MELD score (33). Specifically, sarcopenia is associated with post-LT infectious complications and sepsis-related mortality (35). Sarcopenia is diagnosed based on low muscle mass plus either low muscle strength or low physical performance (150). Modalities such as dual X-ray absorptiometry, bioimpedance analysis, handgrip strength, and gait speed have been used in diagnosis of sarcopenia. However, measurements using dual X-ray absorptiometry and bioimpedance analysis in cirrhotic patients specifically may be distorted by fluid retention (151). Additionally, diminished gait speed and handgrip strength may be due to underlying confusion from hepatic encephalopathy and not necessarily a result of diminished muscle mass (152). Measurement of muscle mass by MRI or CT are gold standards for measuring muscle mass in research (150). Due to multiple modalities used in diagnosing sarcopenia, current literature yields heterogeneous results on assessment of sarcopenia.
Physical activity should be assessed to estimate functional capacity. Metabolic equivalent tasks (METs) are frequently used to assess functional status because they are simple to apply based on the ability of potential recipients to carry out certain tasks. One MET is considered the resting oxygen consumption of a 40-year-old 70 kg man (153). In patients unable to perform 4 METs of work, the preoperative risk is increased (154-156). Table 4 categorizes functional capacity based on METs. Frailty was very prevalent in liver transplant candidates and as frailty score increases waitlist mortality worsened (146). Frailty usually worsens 3 months after LT so intense exercise programs are required pre- and post-transplant to improve endurance (157). Physical activity improves frailty but physical activity was lower in patients awaiting liver transplant and was known to increase portal pressure and increase variceal bleeding (158). Also, a 12-week course of adapted physical activity has improved muscle strength, 6-min walk distance and the ventilatory threshold power in waitlist candidates (159). Supervised aerobic and resistance training is shown to improve physical conditioning and quality in post liver transplant patients (160).
Nutritional intervention should be a focus for treating sarcopenia in cirrhotic patients awaiting LT. The European Society for Parenteral and Enteral Nutrition (ESPEN) recommends a target intake of 35–40 kcal/kg/day and 1.2–1.5 g/kg/day of protein (34). In patients with sarcopenia and hepatic encephalopathy, protein restriction is not recommended (161). In fact, protein restriction in liver transplant candidates is associated with higher mortality while on the waitlist (162). Due to impairments in liver function, patients with cirrhosis have inadequate glycogen stores. To counter the accelerated starvation state in these patients, small, frequent meals and a late evening snack consisting of 50 grams of complex carbohydrates are suggested (162,163). Per a 2016 study by Sinclair et al., testosterone supplementation may safely increase muscle and bone mass in cirrhotic males with sarcopenia and low testosterone levels (164). However, there is currently no treatment directed at cirrhotic patients with sarcopenia. A 2013 review of sarcopenia in the post-LT period attributed unresolved sarcopenia to use of immunosuppressive agents such as mammalian target of rapamycin (mTOR) and CNIs, which can impair skeletal muscle growth, repeated hospitalizations, renal impairment, and infectious complications (165).

Full table
Portal venous thrombosis
Obesity and diabetes are highly prevalent in NASH cirrhosis and are well-known risk factors for vascular thrombosis. Additionally, obesity and diabetes are independent risk factors for developing a pre-transplant portal vein thrombosis (PVT) in liver transplant candidates (166,167). According to Agbim et al., NASH transplant recipients with PVT had a 37% increased risk of graft failure and 31% increased risk of overall death when compared with NASH transplant recipients without PVT at the time of transplant. This difference in graft and patient survival was most pronounced in the first 90 days following LT (36).
Recent evidence suggests that NAFLD mechanistically alters coagulation independent of abdominal adiposity and metabolic syndrome (168). Chronic liver steatosis in NASH patients is associated with an increase in the activity of clotting factor VII, plasminogen activator inhibitor-1 activity and antigen and a decrease in tissue-type plasminogen activator (t-PA) activity (169). In patients with NASH, factor VIII levels seem to be higher and pro C levels seem to be lower, leading to an imbalance in coagulation status (170). Stine et al. reviewed the data of patients who received LT between January 01, 2003 and December 31, 2012 from the United Network for Organ Sharing organization and found that 6.3% patients receiving LT had PVT and 12.0% of those patients had NASH (171). Montenovo et al. and noted that presence of portal venous thrombosis while on the waitlist or at the time of transplant lead to worse patient and graft survival in the post-liver transplant period PVT was also an independent risk factor for being removed from the waitlist (167). Martino et al. studied a total of 465 patients and noted that waitlist mortality was higher in patients with NASH compared to other liver diseases but portal venous thrombosis did not affect waitlist mortality (37). A randomized controlled trial proved that a 12-month course of enoxaparin was effective in preventing portal venous thrombosis in patients with cirrhosis and also improved decompensation and survival rates (172). The use of transjugular intrahepatic portosystemic shunt (TIPS) may be a second-line treatment for PVT if anticoagulation fails, however the data is scarce (173).
PVT poses a technical challenge during LT. The extent of portal vein occlusion can lead to further problems in the post-LT period. Restoring portal blood flow to the allograft is essential for successful transplantation and recovery of liver function (174,175).
Immunosuppressants
Post-transplant metabolic syndrome is very common in NASH patients and is accentuated using immunosuppressive agents. Optimization of dose of immunosuppressive agents improve patient and graft survival. Steroid free regimens are known to decrease diabetes, hyperlipidemia, cytomegalovirus infections but no difference in patient and graft survival, renal insufficiency, hypertension, neurological disorders and infectious complications were noted (102). CNI use is associated with diabetes, hypertriglyceridemia and obesity in post-transplant patients (176). Hypertension and hyperlipidemia are more common in patients using cyclosporine compared to tacrolimus (177). Lower tacrolimus trough concentrations within the first month after LT were associated with less renal impairment at 1 year with no significant influence on acute rejection compared to conventional tacrolimus trough levels (178). But tacrolimus is known to increase NASH after liver transplant (179).
Recent systematic review of 12 studies showed prevalence of de novo NAFLD was 26% and prevalence of NASH was 2%. Highest prevalence of de novo NAFLD were found in patients taking tacrolimus (180). Both cyclosporine and tacrolimus regimen use cause increased risk of cardiovascular events compared to non-cyclosporine regimens (127). Post-transplant deaths, re-transplantation rate was higher in cyclosporine group compared to tacrolimus group (181). The utility of cyclosporine based regimen is of historical interest only. Mycophenolate and mammalian target of rapamycin (mTOR) inhibitors were used to decrease the use of tacrolimus frequently in post-transplant period to decrease metabolic complications (182). Sirolimus-based immunosuppression is associated with a significantly higher risk of NODAT than other immunosuppressants as noted earlier.
Conclusions
Management of NASH in the peritransplant period possesses unique challenges to providers involved in the care of these patients due to its associated comorbidities such as type 2 diabetes, obesity, metabolic syndrome, cardiovascular diseases and CKD. Optimal selection of transplant candidates with NASH involves stringent cardiac evaluation with low threshold for cardiac angiogram particularly in those with high risk CAD history even in the face of normal cardiac stress testing. Pretransplant diabetes is associated with inferior post-operative outcomes and increased resource utilization after LT, and as such a strict control of diabetes using a multidisciplinary approach involving primary care physician, endocrinologist, and dietician combined with a structured weight loss program is of paramount importance for obtaining an optimal outcome in these high-risk patients. Nutritional intervention should be a focus for treating sarcopenia in cirrhotic patients awaiting LT with focus on high protein intake. Frailty is predictor of poor post-transplant outcome, and supervised exercise program should be considered in high risk patients with poor functional capacity. Consideration should be given for early intervention with modification of immunosuppression regimen to protect renal function in those patients with baseline renal dysfunction.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Noureddin M, Vipani A, Bresee C, et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol 2018;113:1649-59. [Crossref] [PubMed]
- Cholankeril G, Wong RJ, Hu M, et al. Liver Transplantation for Nonalcoholic Steatohepatitis in the US: Temporal Trends and Outcomes. Dig Dis Sci 2017;62:2915-22. [Crossref] [PubMed]
- Axley P, Ahmed Z, Arora S, et al. NASH Is the Most Rapidly Growing Etiology for Acute-on-Chronic Liver Failure-Related Hospitalization and Disease Burden in the United States: A Population-Based Study. Liver Transpl 2019;25:695-705. [Crossref] [PubMed]
- Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188-95. [Crossref] [PubMed]
- Golabi P, Bush H, Stepanova M, et al. Liver Transplantation (LT) for Cryptogenic Cirrhosis (CC) and Nonalcoholic Steatohepatitis (NASH) Cirrhosis: Data from the Scientific Registry of Transplant Recipients (SRTR): 1994 to 2016. Medicine (Baltimore) 2018;97:e11518. [Crossref] [PubMed]
- Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557-65. [Crossref] [PubMed]
- Thuluvath PJ, Hanish S, Savva Y. Waiting List Mortality and Transplant Rates for NASH Cirrhosis When Compared With Cryptogenic, Alcoholic, or AIH Cirrhosis. Transplantation 2019;103:113-21. [Crossref] [PubMed]
- Bonora E, Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol 2012;9:372-81. [Crossref] [PubMed]
- Younossi ZM, Marchesini G, Pinto-Cortez H, et al. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation 2019;103:22-7. [Crossref] [PubMed]
- Haldar D, Kern B, Hodson J, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol 2019;71:313-22. [Crossref] [PubMed]
- Agopian VG, Kaldas FM, Hong JC, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg 2012;256:624-33. [Crossref] [PubMed]
- Kennedy C, Redden D, Gray S, et al. Equivalent survival following liver transplantation in patients with non-alcoholic steatohepatitis compared with patients with other liver diseases. HPB (Oxford) 2012;14:625-34. [Crossref] [PubMed]
- Vanwagner LB, Bhave M, Te HS, et al. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology 2012;56:1741-50. [Crossref] [PubMed]
- Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transpl 2012;18:29-37. [Crossref] [PubMed]
- Barritt ASt, Dellon ES, Kozlowski T, et al. The influence of nonalcoholic fatty liver disease and its associated comorbidities on liver transplant outcomes. J Clin Gastroenterol 2011;45:372-8. [Crossref] [PubMed]
- Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249-53. [Crossref] [PubMed]
- Yalamanchili K, Saadeh S, Klintmalm GB, et al. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl 2010;16:431-9. [PubMed]
- Malik SM, deVera ME, Fontes P, et al. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant 2009;9:782-93. [Crossref] [PubMed]
- Bhagat V, Mindikoglu AL, Nudo CG, et al. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl 2009;15:1814-20. [Crossref] [PubMed]
- Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology 2002;35:105-9. [Crossref] [PubMed]
- Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59:1144-65. [Crossref] [PubMed]
- Barone M, Viggiani MT, Losurdo G, et al. Systematic review with meta-analysis: post-operative complications and mortality risk in liver transplant candidates with obesity. Aliment Pharmacol Ther 2017;46:236-45. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address eee. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol 2016;64:433-85. [Crossref] [PubMed]
- Hoehn RS, Singhal A, Wima K, et al. Effect of pretransplant diabetes on short-term outcomes after liver transplantation: a national cohort study. Liver Int 2015;35:1902-9. [Crossref] [PubMed]
- Nishida T. Diagnosis and Clinical Implications of Diabetes in Liver Cirrhosis: A Focus on the Oral Glucose Tolerance Test. J Endocr Soc 2017;1:886-96. [Crossref] [PubMed]
- Ammori JB, Sigakis M, Englesbe MJ, et al. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res 2007;140:227-33. [Crossref] [PubMed]
- Zhang QY, Zhang QF, Zhang DZ. The Impact of Steatosis on the Outcome of Liver Transplantation: A Meta-Analysis. Biomed Res Int 2019;2019:3962785. [PubMed]
- Piazza NA, Singal AK. Frequency of Cardiovascular Events and Effect on Survival in Liver Transplant Recipients for Cirrhosis Due to Alcoholic or Nonalcoholic Steatohepatitis. Exp Clin Transplant 2016;14:79-85. [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57. [Crossref] [PubMed]
- Thongprayoon C, Kaewput W, Thamcharoen N, et al. Incidence and Impact of Acute Kidney Injury after Liver Transplantation: A Meta-Analysis. J Clin Med 2019.8. [PubMed]
- Nakamuta M, Morizono S, Soejima Y, et al. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation 2005;80:608-12. [Crossref] [PubMed]
- VanWagner LB, Lapin B, Skaro AI, et al. Impact of renal impairment on cardiovascular disease mortality after liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Int 2015;35:2575-83. [Crossref] [PubMed]
- Lai JC, Dodge JL, Sen S, et al. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology 2016;63:574-80. [Crossref] [PubMed]
- Plauth M, Cabre E, Riggio O, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr 2006;25:285-94. [Crossref] [PubMed]
- Krell RW, Kaul DR, Martin AR, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl 2013;19:1396-402. [Crossref] [PubMed]
- Agbim U, Jiang Y, Kedia SK, et al. Impact of Nonmalignant Portal Vein Thrombosis in Transplant Recipients With Nonalcoholic Steatohepatitis. Liver Transpl 2019;25:68-78. [Crossref] [PubMed]
- Martino RB, Waisberg DR, Dias APM, et al. Stratifying Mortality in a Model for End-Stage Liver Disease Waiting List: A Brazilian Single-Center Study. Transplant Proc 2018;50:758-61. [Crossref] [PubMed]
- Eshraghian A, Nikeghbalian S, Kazemi K, et al. Portal vein thrombosis in patients with liver cirrhosis and its impact on early and long-term outcomes after liver transplantation. Int J Clin Pract 2018.e13309. [PubMed]
- Wang X, Li J, Riaz DR, et al. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:394-402 e1.
- Dick AA, Spitzer AL, Seifert CF, et al. Liver transplantation at the extremes of the body mass index. Liver Transpl 2009;15:968-77. [Crossref] [PubMed]
- Beckmann S, Drent G, Ruppar T, et al. Body weight parameters are related to morbidity and mortality after liver transplantation - A systematic review and meta-analysis. Transplantation 2019. [PubMed]
- Dare AJ, Plank LD, Phillips AR, et al. Additive effect of pretransplant obesity, diabetes, and cardiovascular risk factors on outcomes after liver transplantation. Liver Transpl 2014;20:281-90. [Crossref] [PubMed]
- Lieber SR, Lee RA, Jiang Y, et al. The impact of post-transplant diabetes mellitus on liver transplant outcomes. Clin Transplant 2019;33:e13554. [Crossref] [PubMed]
- Thuluvath AJ, Chen PH, Thuluvath PJ, et al. Poor Survival After Retransplantation in NASH Cirrhosis. Transplantation 2019;103:101-8. [Crossref] [PubMed]
- Bejaoui M, Pantazi E, Folch-Puy E, et al. Emerging concepts in liver graft preservation. World J Gastroenterol 2015;21:396-407. [Crossref] [PubMed]
- Orman ES, Barritt ASt, Wheeler SB, et al. Declining liver utilization for transplantation in the United States and the impact of donation after cardiac death. Liver Transpl 2013;19:59-68. [Crossref] [PubMed]
- Miyaaki H, Miuma S, Taura N, et al. Risk Factors and Clinical Course for Liver Steatosis or Nonalcoholic Steatohepatitis After Living Donor Liver Transplantation. Transplantation 2019;103:109-12. [Crossref] [PubMed]
- Croome KP, Lee DD, Croome S, et al. The impact of postreperfusion syndrome during liver transplantation using livers with significant macrosteatosis. Am J Transplant 2019;19:2550-9. [Crossref] [PubMed]
- Urena MA, Moreno Gonzalez E, Romero CJ, et al. An approach to the rational use of steatotic donor livers in liver transplantation. Hepatogastroenterology 1999;46:1164-73. [PubMed]
- Wong TC, Fung JY, Chok KS, et al. Excellent outcomes of liver transplantation using severely steatotic grafts from brain-dead donors. Liver Transpl 2016;22:226-36. [Crossref] [PubMed]
- Şeker M, Erol C, Sevmis S, et al. Comparison of CT methods for determining graft steatosis in living donor liver transplantation. Abdom Radiol (NY) 2019;44:2418-29. [Crossref] [PubMed]
- Satkunasingham J, Nik HH, Fischer S, et al. Can negligible hepatic steatosis determined by magnetic resonance imaging-proton density fat fraction obviate the need for liver biopsy in potential liver donors? Liver Transpl 2018;24:470-7. [Crossref] [PubMed]
- Zheng D, Guo Z, Schroder PM, et al. Accuracy of MR Imaging and MR Spectroscopy for Detection and Quantification of Hepatic Steatosis in Living Liver Donors: A Meta-Analysis. Radiology 2017;282:92-102. [Crossref] [PubMed]
- Jun MJ, Shim JH, Kim SY, et al. Clinical implications of preoperative and intraoperative liver biopsies for evaluating donor steatosis in living related liver transplantation. Liver Transpl 2014;20:437-45. [Crossref] [PubMed]
- Boteon YL, Attard J, Boteon A, et al. Manipulation of Lipid Metabolism During Normothermic Machine Perfusion: Effect of Defatting Therapies on Donor Liver Functional Recovery. Liver Transpl 2019;25:1007-22. [Crossref] [PubMed]
- Berzigotti A, Albillos A, Villanueva C, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology 2017;65:1293-305. [Crossref] [PubMed]
- Idriss R, Hasse J, Wu T, et al. Impact of Prior Bariatric Surgery on Perioperative Liver Transplant Outcomes. Liver Transpl 2019;25:217-27. [Crossref] [PubMed]
- Diwan TS, Rice TC, Heimbach JK, et al. Liver Transplantation and Bariatric Surgery: Timing and Outcomes. Liver Transpl 2018;24:1280-7. [Crossref] [PubMed]
- Sharpton SR, Terrault NA. Prior Bariatric Surgery in Liver Transplant Candidates: Unforeseen Consequences? Liver Transpl 2019;25:203-4. [Crossref] [PubMed]
- Singhal A, Wilson GC, Wima K, et al. Impact of recipient morbid obesity on outcomes after liver transplantation. Transpl Int 2015;28:148-55. [Crossref] [PubMed]
- Boin IF, Almeida LV, Udo EY, et al. Survival analysis of obese patients undergoing liver transplantation. Transplant Proc 2007;39:3225-7. [Crossref] [PubMed]
- Braunfeld MY, Chan S, Pregler J, et al. Liver transplantation in the morbidly obese. J Clin Anesth 1996;8:585-90. [Crossref] [PubMed]
- Conzen KD, Vachharajani N, Collins KM, et al. Morbid obesity in liver transplant recipients adversely affects longterm graft and patient survival in a single-institution analysis. HPB (Oxford) 2015;17:251-7. [Crossref] [PubMed]
- Fujikawa T, Fujita S, Mizuno S, et al. Clinical and financial impact of obesity on the outcome of liver transplantation. Transplant Proc 2006;38:3612-4. [Crossref] [PubMed]
- Hakeem AR, Cockbain AJ, Raza SS, et al. Increased morbidity in overweight and obese liver transplant recipients: a single-center experience of 1325 patients from the United Kingdom. Liver Transpl 2013;19:551-62. [Crossref] [PubMed]
- Hillingsø JG, Wettergren A, Hyoudo M, et al. Obesity increases mortality in liver transplantation--the Danish experience. Transpl Int 2005;18:1231-5. [Crossref] [PubMed]
- LaMattina JC, Foley DP, Fernandez LA, et al. Complications associated with liver transplantation in the obese recipient. Clin Transplant 2012;26:910-8. [Crossref] [PubMed]
- Leonard J, Heimbach JK, Malinchoc M, et al. The impact of obesity on long-term outcomes in liver transplant recipients-results of the NIDDK liver transplant database. Am J Transplant 2008;8:667-72. [Crossref] [PubMed]
- Mathur A, Franco ES, Leone JP, et al. Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma. HPB (Oxford) 2013;15:504-10. [Crossref] [PubMed]
- Nair S, Cohen DB, Cohen MP, et al. Postoperative morbidity, mortality, costs, and long-term survival in severely obese patients undergoing orthotopic liver transplantation. Am J Gastroenterol 2001;96:842-5. [Crossref] [PubMed]
- Perez-Protto SE, Quintini C, Reynolds LF, et al. Comparable graft and patient survival in lean and obese liver transplant recipients. Liver Transpl 2013;19:907-15. [Crossref] [PubMed]
- Sawyer RG, Pelletier SJ, Pruett TL. Increased early morbidity and mortality with acceptable long-term function in severely obese patients undergoing liver transplantation. Clin Transplant 1999;13:126-30. [Crossref] [PubMed]
- Schlansky B, Naugler WE, Orloff SL, et al. Higher Mortality and Survival Benefit in Obese Patients Awaiting Liver Transplantation. Transplantation 2016;100:2648-55. [Crossref] [PubMed]
- Singal AK, Kamath PS, Francisco Ziller N, et al. Nutritional status of patients with alcoholic cirrhosis undergoing liver transplantation: time trends and impact on survival. Transpl Int 2013;26:788-94. [Crossref] [PubMed]
- Werneck M, Afonso RC, Coelho GR, et al. Obese and nonobese recipients had similar need for ventilatory support after liver transplantation. Transplant Proc 2011;43:165-9. [Crossref] [PubMed]
- Bambha KM, Dodge JL, Gralla J, et al. Low, rather than high, body mass index confers increased risk for post-liver transplant death and graft loss: Risk modulated by model for end-stage liver disease. Liver Transpl 2015;21:1286-94. [Crossref] [PubMed]
- Beal EW, Tumin D, Conteh LF, et al. Impact of Recipient and Donor Obesity Match on the Outcomes of Liver Transplantation: All Matches Are Not Perfect. J Transplant 2016;2016:9709430. [Crossref] [PubMed]
- Segev DL, Thompson RE, Locke JE, et al. Prolonged waiting times for liver transplantation in obese patients. Ann Surg 2008;248:863-70. [Crossref] [PubMed]
- Pelletier SJ, Schaubel DE, Wei G, et al. Effect of body mass index on the survival benefit of liver transplantation. Liver Transpl 2007;13:1678-83. [Crossref] [PubMed]
- Orci LA, Majno PE, Berney T, et al. The impact of wait list body mass index changes on the outcome after liver transplantation. Transpl Int 2013;26:170-6. [Crossref] [PubMed]
- Heimbach JK, Watt KD, Poterucha JJ, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant 2013;13:363-8. [Crossref] [PubMed]
- Idowu MO, Chhatrala R, Siddiqui MB, et al. De novo hepatic steatosis drives atherogenic risk in liver transplantation recipients. Liver Transpl 2015;21:1395-402. [Crossref] [PubMed]
- Kim H, Lee K, Lee KW, et al. Histologically proven non-alcoholic fatty liver disease and clinically related factors in recipients after liver transplantation. Clin Transplant 2014;28:521-9. [Crossref] [PubMed]
- Richards J, Gunson B, Johnson J, et al. Weight gain and obesity after liver transplantation. Transpl Int 2005;18:461-6. [Crossref] [PubMed]
- Lee YH, Cho Y, Lee BW, et al. Nonalcoholic Fatty Liver Disease in Diabetes. Part I: Epidemiology and Diagnosis. Diabetes Metab J 2019;43:31-45. [Crossref] [PubMed]
- Sinn DH, Kang D, Cho SJ, et al. Lean non-alcoholic fatty liver disease and development of diabetes: A cohort study. Eur J Endocrinol 2019. [Crossref] [PubMed]
- Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:936-44. [Crossref] [PubMed]
- Younossi ZM, Golabi P, de Avila L, et al. The Global Epidemiology of NAFLD and NASH in Patients with type 2 diabetes: A Systematic Review and Meta-analysis. J Hepatol 2019;71:793-801. [Crossref] [PubMed]
- Yang JD, Ahmed F, Mara KC, et al. Diabetes is Associated with Increased Risk of Hepatocellular Carcinoma in Cirrhosis Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Kardashian AA, Dodge JL, Roberts J, et al. Weighing the risks: Morbid obesity and diabetes are associated with increased risk of death on the liver transplant waiting list. Liver Int 2018;38:553-63. [Crossref] [PubMed]
- Silva TE, Ronsoni MF, Schiavon LL. Challenges in diagnosing and monitoring diabetes in patients with chronic liver diseases. Diabetes Metab Syndr 2018;12:431-40. [Crossref] [PubMed]
- Nadelson J, Satapathy SK, Nair S. Glycated Hemoglobin Levels in Patients with Decompensated Cirrhosis. Int J Endocrinol 2016;2016:8390210. [Crossref] [PubMed]
- Liu TL, Barritt AI, Weinberger M, et al. Who Treats Patients with Diabetes and Compensated Cirrhosis. PLoS One 2016;11:e0165574. [Crossref] [PubMed]
- Tokodai K, Karadagi A, Kjaernet F, et al. Characteristics and risk factors for recurrence of nonalcoholic steatohepatitis following liver transplantation. Scand J Gastroenterol 2019;54:233-9. [Crossref] [PubMed]
- Molinari M, Ayloo S, Tsung A, et al. Prediction of Perioperative Mortality of Cadaveric Liver Transplant Recipients during their evaluations. Transplantation 2019;103:e297-307. [Crossref] [PubMed]
- Ong J, Younossi ZM, Reddy V, et al. Cryptogenic cirrhosis and posttransplantation nonalcoholic fatty liver disease. Liver Transpl 2001;7:797-801. [Crossref] [PubMed]
- El Atrache MM, Abouljoud MS, Divine G, et al. Recurrence of non-alcoholic steatohepatitis and cryptogenic cirrhosis following orthotopic liver transplantation in the context of the metabolic syndrome. Clin Transplant 2012;26:E505-12. [Crossref] [PubMed]
- Finkenstedt A, Auer C, Glodny B, et al. Patatin-like phospholipase domain-containing protein 3 rs738409-G in recipients of liver transplants is a risk factor for graft steatosis. Clin Gastroenterol Hepatol 2013;11:1667-72. [Crossref] [PubMed]
- Peláez-Jaramillo MJ, Cardenas-Mojica AA, Gaete PV, et al. Post-Liver Transplantation Diabetes Mellitus: A Review of Relevance and Approach to Treatment. Diabetes Ther 2018;9:521-43. [Crossref] [PubMed]
- Bhat V, Tazari M, Watt KD, et al. New-Onset Diabetes and Preexisting Diabetes Are Associated With Comparable Reduction in Long-Term Survival After Liver Transplant: A Machine Learning Approach. Mayo Clin Proc 2018;93:1794-802. [Crossref] [PubMed]
- Lv C, Zhang Y, Chen X, et al. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes 2015;7:881-90. [Crossref] [PubMed]
- Sgourakis G, Dedemadi G. Corticosteroid-free immunosuppression in liver transplantation: an evidence-based review. World J Gastroenterol 2014;20:10703-14. [Crossref] [PubMed]
- Wallia A, Parikh ND, Molitch ME, et al. Posttransplant hyperglycemia is associated with increased risk of liver allograft rejection. Transplantation 2010;89:222-6. [Crossref] [PubMed]
- Wallia A, Parikh ND, O'Shea-Mahler E, et al. Glycemic control by a glucose management service and infection rates after liver transplantation. Endocr Pract 2011;17:546-51. [Crossref] [PubMed]
- Yoo S, Lee HJ, Lee H, et al. Association Between Perioperative Hyperglycemia or Glucose Variability and Postoperative Acute Kidney Injury After Liver Transplantation: A Retrospective Observational Study. Anesth Analg 2017;124:35-41. [Crossref] [PubMed]
- Galindo RJ, Wallia A. Hyperglycemia and Diabetes Mellitus Following Organ Transplantation. Curr Diab Rep 2016;16:14. [Crossref] [PubMed]
- Wallia A, Illuri V, Molitch ME. Diabetes Care After Transplant: Definitions, Risk Factors, and Clinical Management. Med Clin North Am 2016;100:535-50. [Crossref] [PubMed]
- Hartog H, May CJ, Corbett C, et al. Early occurrence of new-onset diabetes after transplantation is related to type of liver graft and warm ischaemic injury. Liver Int 2015;35:1739-47. [Crossref] [PubMed]
- Paka P, Lieber SR, Lee RA, et al. Perioperative glucose management and outcomes in liver transplant recipients: A qualitative systematic review. World J Transplant 2018;8:75-83. [Crossref] [PubMed]
- Orman ES, Mayorga ME, Wheeler SB, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl 2015;21:1040-50. [Crossref] [PubMed]
- Shanbhogue RL, Bistrian BR, Jenkins RL, et al. Increased protein catabolism without hypermetabolism after human orthotopic liver transplantation. Surgery 1987;101:146-9. [PubMed]
- Ferreira SC, Penaforte FR, Cardoso AS, et al. Eating behaviour patterns are associated with excessive weight gain after liver transplantation. J Hum Nutr Diet 2019. [Crossref] [PubMed]
- Singhvi A, Sadowsky HS, Cohen A, et al. Resting and Exercise Energy Metabolism After Liver Transplantation for Nonalcoholic Steatohepatitis. Transplant Direct 2017;3:e188. [Crossref] [PubMed]
- Lalama MA, Saloum Y. Nutrition, fluid, and electrolytes in chronic liver disease. Clin Liver Dis (Hoboken) 2016;7:18-20. [Crossref] [PubMed]
- Moura Neto A, Bovi TG, Righetto CM, et al. Clinical Profile of Patients With Diabetes Mellitus and Liver Transplantation: Results After a Multidisciplinary Team Intervention. Transplant Proc 2018;50:784-7. [Crossref] [PubMed]
- Patel SS, Nabi E, Guzman L, et al. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transpl 2018;24:333-42. [Crossref] [PubMed]
- Nagai S, Collins K, Chau LC, et al. Increased Risk of Death in First Year After Liver Transplantation Among Patients with Nonalcoholic Steatohepatitis vs Liver Disease of Other Etiologies. Clin Gastroenterol Hepatol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Nandhakumar A, McCluskey SA, Srinivas C, et al. Liver transplantation: Advances and perioperative care. Indian J Anaesth 2012;56:326-35. [Crossref] [PubMed]
- Harinstein ME, Flaherty JD, Ansari AH, et al. Predictive value of dobutamine stress echocardiography for coronary artery disease detection in liver transplant candidates. Am J Transplant 2008;8:1523-8. [Crossref] [PubMed]
- Soldera J, Camazzola F, Rodriguez S, et al. Dobutamine stress echocardiography, myocardial perfusion scintigraphy, invasive coronary angiography, and post-liver transplantation events: Systematic review and meta-analysis. Clin Transplant 2018;32:e13222. [Crossref] [PubMed]
- Vaduganathan P, He ZX, Raghavan C, et al. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996;28:543-50. [Crossref] [PubMed]
- Snipelisky D, Levy M, Shapiro B. Utility of dobutamine stress echocardiography as part of the pre-liver transplant evaluation: an evaluation of its efficacy. Clin Cardiol 2014;37:468-72. [Crossref] [PubMed]
- Pillarisetti J, Patel P, Duthuluru S, et al. Cardiac catheterization in patients with end-stage liver disease: safety and outcomes. Catheter Cardiovasc Interv 2011;77:45-8. [Crossref] [PubMed]
- van Kuijk JP, Flu WJ, Schouten O, et al. Timing of noncardiac surgery after coronary artery stenting with bare metal or drug-eluting stents. Am J Cardiol 2009;104:1229-34. [Crossref] [PubMed]
- Liu H, Jayakumar S, Traboulsi M, et al. Cirrhotic cardiomyopathy: Implications for liver transplantation. Liver Transpl 2017;23:826-35. [Crossref] [PubMed]
- Luca L, Westbrook R, Tsochatzis EA. Metabolic and cardiovascular complications in the liver transplant recipient. Ann Gastroenterol 2015;28:183-92. [PubMed]
- Fussner LA, Heimbach JK, Fan C, et al. Cardiovascular disease after liver transplantation: When, What, and Who Is at Risk. Liver Transpl 2015;21:889-96. [Crossref] [PubMed]
- Satapathy SK, Vanatta JM, Helmick RA, et al. Outcome of Liver Transplant Recipients With Revascularized Coronary Artery Disease: A Comparative Analysis With and Without Cardiovascular Risk Factors. Transplantation 2017;101:793-803. [Crossref] [PubMed]
- Kalisvaart M, Schlegel A, Umbro I, et al. The AKI Prediction Score: a new prediction model for acute kidney injury after liver transplantation. HPB (Oxford) 2019. [Epub ahead of print].
- Tan L, Yang Y, Ma G, et al. Early acute kidney injury after liver transplantation in patients with normal preoperative renal function. Clin Res Hepatol Gastroenterol 2019;43:475-82. [Crossref] [PubMed]
- Houlihan DD, Armstrong MJ, Davidov Y, et al. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: time to reconsider immunosuppression regimens? Liver Transpl 2011;17:1292-8. [Crossref] [PubMed]
- Xu HW, Hsu YC, Chang CH, et al. High FIB-4 index as an independent risk factor of prevalent chronic kidney disease in patients with nonalcoholic fatty liver disease. Hepatol Int 2016;10:340-6. [Crossref] [PubMed]
- Targher G, Bertolini L, Rodella S, et al. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol 2010;5:2166-71. [Crossref] [PubMed]
- Musso G, Gambino R, Tabibian JH, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014;11:e1001680. [Crossref] [PubMed]
- Chinnadurai R, Chrysochou C, Kalra PA. Increased Risk for Cardiovascular Events in Patients with Diabetic Kidney Disease and Non-Alcoholic Fatty Liver Disease. Nephron 2019;141:24-30. [Crossref] [PubMed]
- Paik J, Golabi P, Younoszai Z, et al. Chronic kidney disease is independently associated with increased mortality in patients with nonalcoholic fatty liver disease. Liver Int 2019;39:342-52. [Crossref] [PubMed]
- Fussner LA, Charlton MR, Heimbach JK, et al. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int 2014;34:1259-66. [Crossref] [PubMed]
- Singal AK, Hasanin M, Kaif M, et al. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous Liver Kidney Transplantation in the United States. Transplantation 2016;100:607-12. [Crossref] [PubMed]
- Molnar MZ, Joglekar K, Jiang Y, et al. Association of Pretransplant Renal Function With Liver Graft and Patient Survival After Liver Transplantation in Patients With Nonalcoholic Steatohepatitis. Liver Transpl 2019;25:399-410. [Crossref] [PubMed]
- Santos SH, Andrade JM, Fernandes LR, et al. Oral Angiotensin-(1-7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-kappaB in rats fed with high-fat diet. Peptides 2013;46:47-52. [Crossref] [PubMed]
- Pelusi S, Petta S, Rosso C, et al. Renin-Angiotensin System Inhibitors, Type 2 Diabetes and Fibrosis Progression: An Observational Study in Patients with Nonalcoholic Fatty Liver Disease. PLoS One 2016;11:e0163069. [Crossref] [PubMed]
- Alam S, Kabir J, Mustafa G, et al. Effect of telmisartan on histological activity and fibrosis of non-alcoholic steatohepatitis: A 1-year randomized control trial. Saudi J Gastroenterol 2016;22:69-76. [Crossref] [PubMed]
- Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology 2011;54:1610-9. [Crossref] [PubMed]
- Leporini C, Pisano A, Russo E, et al. Effect of pentoxifylline on renal outcomes in chronic kidney disease patients: A systematic review and meta-analysis. Pharmacol Res 2016;107:315-32. [Crossref] [PubMed]
- Musso G, De Michieli F, Bongiovanni D, et al. New Pharmacologic Agents That Target Inflammation and Fibrosis in Nonalcoholic Steatohepatitis-Related Kidney Disease. Clin Gastroenterol Hepatol 2017;15:972-85. [Crossref] [PubMed]
- Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870-9. [Crossref] [PubMed]
- Kaido T, Tamai Y, Hamaguchi Y, et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition 2017;33:195-8. [Crossref] [PubMed]
- Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 2002;57:M772-7. [Crossref] [PubMed]
- Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc 2008;56:1710-5. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Kallwitz ER. Sarcopenia and liver transplant: The relevance of too little muscle mass. World J Gastroenterol 2015;21:10982-93. [Crossref] [PubMed]
- Uchiyama H. Sarcopenia in liver transplant recipients: its relevance to peritransplant morbidity and mortality. Hepatobiliary Surg Nutr 2017;6:196-9. [Crossref] [PubMed]
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Developed in collaboration with the American College of Surgeons, American Society of Anesthesiologists, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Anesthesiologists, and Society of Vascular Medicine Endorsed by the Society of Hospital Medicine. J Nucl Cardiol 2015;22:162-215. [Crossref] [PubMed]
- Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med 1999;159:2185-92. [Crossref] [PubMed]
- Tsiouris A, Horst HM, Paone G, et al. Preoperative risk stratification for thoracic surgery using the American College of Surgeons National Surgical Quality Improvement Program data set: functional status predicts morbidity and mortality. J Surg Res 2012;177:1-6. [Crossref] [PubMed]
- Goswami S, Brady JE, Jordan DA, et al. Intraoperative cardiac arrests in adults undergoing noncardiac surgery: incidence, risk factors, and survival outcome. Anesthesiology 2012;117:1018-26. [Crossref] [PubMed]
- Lai JC, Segev DL, McCulloch CE, et al. Physical frailty after liver transplantation. Am J Transplant 2018;18:1986-94. [Crossref] [PubMed]
- García-Pagàn JC, Santos C, Barbera JA, et al. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology 1996;111:1300-6. [Crossref] [PubMed]
- Debette-Gratien M, Tabouret T, Antonini MT, et al. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation 2015;99:145-50. [Crossref] [PubMed]
- Moya-Nájera D, Moya-Herraiz A, Compte-Torrero L, et al. Combined resistance and endurance training at a moderate-to-high intensity improves physical condition and quality of life in liver transplant patients. Liver Transpl 2017;23:1273-81. [Crossref] [PubMed]
- Córdoba J, Lopez-Hellin J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol 2004;41:38-43. [Crossref] [PubMed]
- Ney M, Abraldes JG, Ma M, et al. Insufficient Protein Intake Is Associated With Increased Mortality in 630 Patients With Cirrhosis Awaiting Liver Transplantation. Nutr Clin Pract 2015;30:530-6. [Crossref] [PubMed]
- Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol 2012;27:430-41. [Crossref] [PubMed]
- Sinclair M, Grossmann M, Hoermann R, et al. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol 2016;65:906-13. [Crossref] [PubMed]
- Dasarathy S. Posttransplant sarcopenia: an underrecognized early consequence of liver transplantation. Dig Dis Sci 2013;58:3103-11. [Crossref] [PubMed]
- Targher G, Chonchol M, Miele L, et al. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost 2009;35:277-87. [Crossref] [PubMed]
- Montenovo M, Rahnemai-Azar A, Reyes J, et al. Clinical Impact and Risk Factors of Portal Vein Thrombosis for Patients on Wait List for Liver Transplant. Exp Clin Transplant 2018;16:166-71. [PubMed]
- Kotronen A, Joutsi-Korhonen L, Sevastianova K, et al. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int 2011;31:176-83. [Crossref] [PubMed]
- Cigolini M, Targher G, Agostino G, et al. Liver steatosis and its relation to plasma haemostatic factors in apparently healthy men--role of the metabolic syndrome. Thromb Haemost 1996;76:69-73. [Crossref] [PubMed]
- Tripodi A, Fracanzani AL, Primignani M, et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol 2014;61:148-54. [Crossref] [PubMed]
- Stine JG, Shah NL, Argo CK, et al. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl 2015;21:1016-21. [Crossref] [PubMed]
- Villa E, Camma C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253-60.e4. [Crossref] [PubMed]
- Rössle M, Bausch B, Klinger C. Therapy Algorithm for Portal Vein Thrombosis in Liver Cirrhosis: The Internist's Point of View. Viszeralmedizin 2014;30:401-8. [Crossref] [PubMed]
- Francoz C, Valla D, Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol 2012;57:203-12. [Crossref] [PubMed]
- Wissing KM, Pipeleers L. Obesity, metabolic syndrome and diabetes mellitus after renal transplantation: prevention and treatment. Transplant Rev (Orlando) 2014;28:37-46. [Crossref] [PubMed]
- Andrade AR, Bittencourt PL, Codes L, et al. New Onset Diabetes and Non-Alcoholic Fatty Liver Disease after Liver Transplantation. Ann Hepatol 2017;16:932-40. [Crossref]
- Bianchi G, Marchesini G, Marzocchi R, et al. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl 2008;14:1648-54. [Crossref] [PubMed]
- Rodríguez-Perálvarez M, Germani G, Darius T, et al. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant 2012;12:2797-814. [Crossref] [PubMed]
- Dumortier J, Giostra E, Belbouab S, et al. Non-alcoholic fatty liver disease in liver transplant recipients: another story of "seed and soil". Am J Gastroenterol 2010;105:613-20. [Crossref] [PubMed]
- Losurdo G, Castellaneta A, Rendina M, et al. Systematic review with meta-analysis: de novo non-alcoholic fatty liver disease in liver-transplanted patients. Aliment Pharmacol Ther 2018;47:704-14. [Crossref] [PubMed]
- O'Grady JG, Burroughs A, Hardy P, et al. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet 2002;360:1119-25. [Crossref] [PubMed]
- Khan RS, Newsome PN. Non-alcoholic fatty liver disease and liver transplantation. Metabolism 2016;65:1208-23. [Crossref] [PubMed]
Cite this article as: Samji NS, Heda R, Satapathy SK. Peri-transplant management of nonalcoholic fatty liver disease in liver transplant candidates . Transl Gastroenterol Hepatol 2020;5:10.


