
Esophageal, gastric cancer and immunotherapy: small steps in the right direction?
Introduction
Esophageal cancer (consisting of both squamous cell carcinoma and adenocarcinoma) is the eighteenth most common malignancy in the US by incidence with 17,650 new cases in 2019, representing 1.0% of all new cancer cases (1). Esophageal cancer accounts for 2.6% of cancer deaths in 2019, with 16,080 succumbing to this disease (1). Nearly 40% of patients present with metastatic disease at diagnosis (1). Rates of new cases and deaths have been falling by nearly 1% annually over the last 10 years (1). While it has a five-year survival of 19.9%, although five-year survival in patients with metastatic disease is 4.8% (1). As of 2016, an estimated 46,477 people were living with esophageal cancer (1).
In the United States, gastric cancer is the fifteenth most common cancer by incidence with 27,510 new cases diagnosed in 2019, representing 1.6% of all new cancers (2). Gastric cancer resulted in 11,140 deaths in 2019, representing 1.8% of cancer deaths (2). Nearly 40% of patients present with metastatic disease at diagnosis (2). Despite a five-year survival of 31.5%, overall, five-year survival in patients with metastatic disease is far less, 5.3% (2). Based on estimates from 2016, approximately 113,054 people were living with gastric cancer in the US (2). Both the rates of new cases and death have declined by 1.5% and 2.1% annually over the last 10 years, respectively (2).
Systemic treatment of advanced, metastatic esophageal, gastroesophageal junction (GEJ), and gastric cancer utilizes a combination of multiple cytotoxic chemotherapeutic agents, although no single, standard of care regimen exists. Combination chemotherapy with a platinum and fluoropyrimidine doublet, such as FOLFOX, CAPOX, cisplatin/5-fluorouracil (5-FU), or cisplatin/capecitabine are common regimens with the addition of Trastuzumab for the treatment of HER2-positive disease (3-7). Other agents like Irinotecan, or taxanes (docetaxel or paclitaxel) can be combined with fluoropyrimidines and/or platinums or ramicurumab, or used as monotherapy for those unfit for combination regimens (8-10).
The use of immune checkpoint inhibitors (ICIs) in the treatment of both hematologic malignancies and solid organ malignancies has been expanding rapidly since the first approval for Ipilimumab in 2011 for the treatment of BRAF-negative metastatic melanoma (11-14). Now, more than 1,000 immunotherapy clinical trials later, we are exploring their uses in countless malignancies in first, second and later-line metastatic disease, as well as in the adjuvant setting. This review article will focus on the use of the currently studied, approved uses and the future roles of these agents in the treatment of cancers of the esophagus, GEJ, and stomach.
Current role and rationale for immunotherapy in the treatment paradigm of esophageal, GEJ, and gastric cancers
Despite the numerous approvals for immunotherapy in other malignancies, gastrointestinal cancers have had limited approvals to date. Currently, there is no role for immunotherapy in the neoadjuvant or adjuvant settings in resectable disease, or in first-line treatment of unresectable locally advanced, recurrent, or metastatic esophageal, gastroesophageal, or gastric cancers. However, a single immunotherapy agent, pembrolizumab, is considered an approved treatment option in subset of patients in either the second-line, third-line, or later-line settings in unresectable locally advanced, recurrent, or metastatic esophageal, gastroesophageal, or gastric cancers. The two subsets of patients with advanced, unresectable, or metastatic solid tumors, who its use could be considered are patients with tumors demonstrating: (I) microsatellite instability-high (MSI-H) or (II) DNA mismatch repair deficient (dMMR). Additional indications for ICI treatment of esophageal, GEJ, and gastric cancers are esophageal, GEJ, or gastric adenocarcinomas with PD-L1 CPS greater than or equal to 1, or esophageal squamous cell carcinoma (ESCC) with CPS greater than or equal to 10.
Similar to many other GI malignancies, Pembrolizumab gained approval for either MSI-H or dMMR unresectable, or metastatic solid tumors in May 2017 (15,16). This approval came in the wake of a study by Le et al. evaluating patients with mismatch repair-deficient malignancies, after this signal was seen in colorectal cancers (15,16). Overall response rates (ORR) was seen in 53% of patients with dMMR malignancies (see Table 1) across 12 different tumor types, including esophageal, GEJ, and gastric cancers (16). Mismatch repair deficiencies are seen in ~1% of esophageal and GEJ cancers and nearly 9% of gastric adenocarcinomas (16).
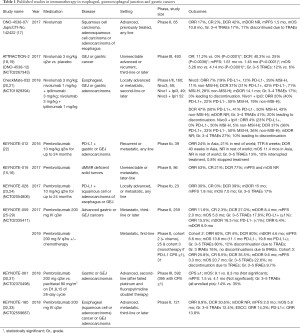
Full table
The use of immunotherapy in adenocarcinoma of the GEJ and stomach began with multinational phase Ib KEYNOTE-012 study (NCT01848834) evaluating safety and tolerability of Pembrolizumab in several solid tumor cohorts, including triple-negative breast cancer, urothelial cancer, head and neck cancer, and advanced gastric cancer, with the results of the gastric cancer cohort published in 2016 (22). This small study revealed early promising results with ORR of 24% in Asia and 22% in patients from the rest of the world (see Table 1) and was generally well tolerated with 13% grade 3 or greater treatment related adverse events (22). Only 10% of patients had to interrupt treatment due to toxicity (22). Due to this signal of efficacy and tolerability shown in KEYNOTE-012, further industry-supported studies evaluating the efficacy of PD-L1 inhibition with Pembrolizumab have been undertaken. KEYNOTE-059 (NCT02335411) is the phase II study in 259 patients with advanced gastric and gastroesophageal adenocarcinoma who had received at least two previous lines of therapy to assess the safety and response rates of pembrolizumab monotherapy (25-27). The study showed promise from a tolerability and activity standpoint (see Table 1). ORR was 11.6% and 17.8% of patients experienced grade 3 or higher TRAEs with 0.8% discontinuing treatment as a result of TRAEs (25-27). The duration of response was 8.4 months overall. However, in patients with PD-L1 positive tumors (greater than or equal to 1% expression), the objective response rate and duration of response was much more promising, at 15.5% and 16.3 months (25-27). Due to the promise seen with immune checkpoint inhibition in GEJ and gastric cancers in these early phase studies, the FDA approved Pembrolizumab for treatment of advanced or metastatic gastric, or GEJ, adenocarcinoma with CPS greater than or equal to 1 with disease progression after two lines of systemic therapy in September 2017 (34). Additionally, the phase III, KEYNOTE-061 (NCT02370498) study is underway to further assess the efficacy of Pembrolizumab (30,31). However, despite this signal for potential impact, pembrolizumab failed to significantly improve overall survival or progression free survival in advanced GEJ or gastric cancers compared with single-agent paclitaxel, despite its better tolerability (see Table 1) (30,31).
The much anticipated results of the phase III study, KEYNOTE-062, of Pembrolizumab in first-line treatment of advanced or metastatic gastric or GEJ adenocarcinoma were recently presented at the 2019 ASCO Annual Meeting (35,36). KEYNOTE-062 assessed the efficacy and safety of Pembrolizumab with and without chemotherapy to chemotherapy alone in first-line advanced, or metastatic, gastric or GEJ cancers (see Table 2). In patients with CPS greater than or equal to 1, Pembrolizumab and chemotherapy did not result in superior mOS (12.5 vs. 11.1 mos; HR 0.85, 95% CI: 0.70–1.03) or mPFS (6.9 vs. 6.4 mos; HR 0.84, 95% CI: 0.70–1.02) compared to chemotherapy alone. However, Pembrolizumab monotherapy was found to have non-inferior mOS compared with chemotherapy (10.6 vs. 11.1 mos; HR 0.91, 95% CI: 0.69–1.18; non-inferiority margin 1.2) despite lower ORR (14.5% vs. 36.8%, respectively) (35,36). However, in patients with strongly positive PD-L1 tumors (CPS ≥10), pembrolizumab monotherapy resulted in a significant improvement in mOS (17.4 vs. 10.8 mos; HR 0.69, 95% CI: 0.49–0.97) compared with chemotherapy (35,36).
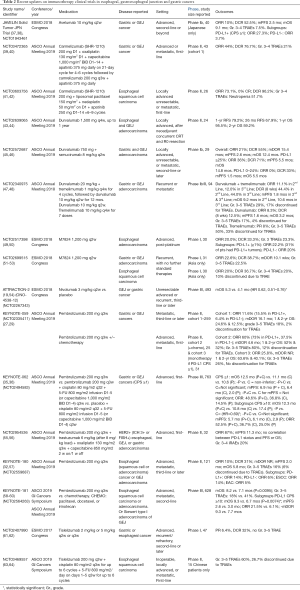
Full table
KEYNOTE-028 was one of the first studies to investigate the safety and activity of Pembrolizumab in the treatment of esophageal carcinoma (23,24). This phase Ib study investigating several solid tumor types, including a cohort of 23 patients with esophageal cancer, 78% exhibiting squamous cell histology, served as an early signal for both safety and efficacy in PD-L1 expressing esophageal carcinomas. ORR was 30% with 17% of patients experiencing grade 3 or higher TRAEs (see Table 1) (23,24). This study was open to any patients with locally advanced, or metastatic esophageal carcinoma, regardless of histologic subtype, who had either failed prior standard therapies or were not candidates for these therapies, with 87% of patients enrolled having received at least 2 lines of prior therapy (23,24). Of note, esophageal cancers exhibit variable rates of PD-L1 depending on the histology of the tumor, with approximately 18% in adenocarcinomas and 44% in squamous cell carcinomas (65,66). Given the potential for a significant population of patients with esophageal cancer who could benefit from immunotherapy, clinical trials were undertaken to assess for safety and efficacy. Next, came KEYNOTE-180 (NCT02559687), that evaluated advanced, metastatic ESCC, esophageal and GEJ adenocarcinomas who had failed at least two prior lines of therapy, regardless of PD-L1 expression status (32,33). The majority of patients enrolled were ESCC (52.1%) and just under half of the patients had PD-L1 positive tumors (47.9%), defined at combined positive score (CPS) greater than or equal to 10%. ORR was just 9.9%, DCR was 30.6% and 12.4% of all patients experiencing grade 3 or higher TRAEs, leading to discontinuation of treatment in 4.1%. Objective response rates were slightly better in subgroups of patients with ESCC (14.3%) and PD-L1-positive tumors (13.8%) (32,33). A recent update of this cohort from KEYNOTE-180 with longer follow-up was presented at 2019 ASCO Annual Meeting, with similar results (discussed in further detail below) (32,57). Furthermore, the results of KEYNOTE-181 (NCT02564263), a phase III study evaluating second-line Pembrolizumab in advanced or metastatic ESCC, EAC, or adenocarcinoma of GEJ support the use of Pembrolizumab monotherapy. In patients with CPS greater than or equal to 10, Pembrolizumab resulted in superior overall survival compared with second-line chemotherapy (9.3 vs. 6.7 mos; HR 0.69, 95% CI: 0.52–0.93), along with lower rates of grade 3–5 TRAEs (18% vs. 41%) (58-60). Median OS for ESCC with CPS greater than or equal to 10 was 10.3 months compared with 6.7 months with chemotherapy, 12-month OS was 48% vs. 23% respectively (58,59). One important question remaining within this study population is the presence of MSI-H, which may dilute these results. As a result of the findings of KEYNOTE-180 and KEYNOTE-181, Pembrolizumab was granted FDA approval as monotherapy for the treatment of recurrent locally-advanced, or metastatic, ESCC with CPS greater than or equal to 10 with disease progression after one or more systemic treatments on July 30, 2019 (67).
Future directions of immunotherapy in esophageal, GEJ and gastric cancers
Although only pembrolizumab is approved for treatment of upper GI tract malignancies, we are privy to a vast array of early phase trials with several ICIs, in larger groups of patients with a variety of diseases. In the subsections below, we will briefly discuss each immunotherapy agent, the current literature and upcoming, large studies that are underway to further establish their individual roles in the treatment of esophageal, GEJ and gastric cancers. Each of these agents is discussed further in Tables 1-3, which outline published studies, recent updates for ongoing clinical trials and upcoming phase II and III clinical trials which are enrolling more than 100 patients but have not published any results to date, respectively.
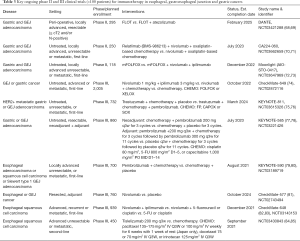
Full table
Atezolizumab
Atezolizumab has found a role in the treatment of several solid tumors, including most notably, extensive small cell lung cancer, and metastatic urothelial carcinoma. However, its role in GI malignancies is much less established. While there are several ongoing small (<100 patients), phase I and II studies that are underway, there has not been any published results for the use of atezolizumab in esophageal, GEJ or gastric cancers. No completed studies small (<100 patients). The largest study underway is the phase II DANTE study (NCT03421288) evaluating the use of peri-operative FLOT vs. FLOT and Atezolizumab in locally advanced, resectable gastric and GEJ adenocarcinoma with expected study completion in February 2025 (68,69).
Avelumab
Avelumab is an anti-PD-L1 monoclonal antibody approved in Europe for gastric cancer since 2017; however, it is currently only approved by the FDA for use in Merkel cell carcinoma in the US. The phase Ib JAVELIN Solid Tumor JPN study evaluated the role of Avelumab in advanced gastric or GEJ cancers after failed first line treatment amongst 40 Japanese patients. It showed limited effectiveness in this setting with ORR of 10% independent of PD-L1 expression with low rates of grade 3–4 TRAEs (7.5%) (37,38). However, subgroup analysis based on PD-L1 expression exhibited higher ORR (27.3%) in patients with CPS scores greater than or equal to 1 (37,38). Despite these findings, no large (>100) patient phase II or III studies are ongoing in the United States.
Camrelizumab (SHR-1210)
Camrelizumab (SHR-1210) is a novel anti-PD-L1 monoclonal antibody whose role in treatment of Hodgkin lymphoma, hepatocellular carcinoma, esophageal, GEJ and gastric cancers is being evaluated by clinical trials. While no large (>100 patient), phase II or III studies are currently underway in the US for these disease states, two small Phase II studies recently reported abstract data at the 2019 ASCO Annual Meeting. The first of these studies is the phase II study (NCT03472365) evaluating camrelizumab in combination with chemotherapy (oxaliplatin and capecitabine) and anti-VEGFR2 therapy (apatinib) in advanced, or metastatic GEJ or gastric cancer in the first-line setting (39,40). This showed early promise with ORR of 44%, DCR of 76.7%, and was generally well tolerated with 21% of patients experiencing grade 3–4 TRAEs (39,40). The second of these phase II studies evaluated the use of first-line Camrelizumab with combination chemotherapy and Apatinib in locally advanced, unresectable, or metastatic, ESCC with robust ORR of 73.1%, DCR of 96.2%. However, this regimen carried with it significant grade 3–4 TRAEs, namely 51.7% neutropenia (41,42). These studies were agnostic of PD-L1 or MSI testing.
Durvalumab
Durvalumab (MEDI4736) is another anti-PD-L1 monoclonal antibody under investigation in upper gastrointestinal malignancies and other solid malignancies. It is not currently used for treatment in any GI malignancy outside of clinical trial. Its only approved uses are in unresectable Stage III non-small cell lung cancer that has not progressed (i.e., maintenance) after concurrent platinum-based chemotherapy and radiation therapy and in locally advanced or metastatic urothelial cancer following progression on platinum-containing chemotherapy or within 12 months of receiving platinum-containing chemotherapy peri-operatively (neoadjuvant or adjuvant). Durvalumab is being investigated in the adjuvant setting as monotherapy, and in combination with another ICI, tremelimumab, or in combination with ramucirumab, a VEGFR2 inhibitor, in advanced gastric and GEJ adenocarcinoma.
The role of durvalumab in locally advanced esophageal and GEJ adenocarcinoma in the adjuvant setting was evaluated in a study out of the Big Ten Consortium with early results presented at the 2019 ASCO Annual Meeting (43,44). The use of durvalumab was evaluated after neoadjuvant, concurrent chemotherapy and radiation and R0 resection in locally advanced disease (43,44). With the addition of durvalumab to standard tri-modality treatment, patients had 1-year relapse-free survival (RFS) of 79.2% and 1-year overall survival of 95.5% with low rates of grade 3 irAEs (12.5%) (43,44). Historically, trimodality therapy resulted in 1-year RFS of 50%. Given the tolerability of durvalumab in the adjuvant setting, and nearly 30% absolute improvement in 1-year RFS, further large studies are needed to assess its safety and efficacy, although none are currently underway in the United States (43,44).
In a phase Ib/II study by Kelly et al. (NCT02340975), the use of Durvalumab and Tremelimumab were evaluated in advanced GEJ and gastric adenocarcinomas after failed systemic treatment, alone and in combination (47,48). Of the 94 patients reported in the 2018 ASCO abstract, 58 patients received a combination of durvalumab and tremelimumab (35% with PD-L1 >1%; outcomes in 27 in second-line; 25 in third-line are reported), 24 received durvalumab alone (38% with PD-L1 >1%) in the second-line setting, and 12 patients received tremelimumab alone (50% PD-L1 >1%) in the second-line setting (47,48). The combination of durvalumab and tremelimumab in both the second-line and third-line setting had modest ORR (11.1 and 12.0, respectively) with limited PFS (1.8 months for in both lines) (47,48). Overall survival was more promising in combination ICI compared with single agent durvalumab (9.2 mos in second-line, 10.6 mos in third-line and 3.2 months in single agent second-line), although combination therapy had higher grade 3–5 TRAEs (29% vs. 17%) with higher discontinuation rates due to TRAEs (17% vs. 4%) compared with Durvalumab alone (47,48). The final results of this study have not been published to date.
Additionally, the combination of durvalumab and the VEGF2 inhibitor, ramucirumab as part of a basket study included a cohort of patients with advanced gastric or gastroesophageal adenocarcinoma (45,46). ORR was modest (21%) amongst all 29 enrolled patients, which was higher (36%) in the 14 patients with ‘high’ PD-L1 expression (greater than or equal to 25% of tumor cells and/or immune cells) and negligible in patients with ‘low’ PD-L1 expression (0%) (45,46). Median PFS (2.6 mos) and median OS (12.4 mos) for all patients was modest, which were both greater in the subgroup with high PD-L1 expression (mPFS 5.5 mos; mOS 14.8 mos) (45,46). Median PFS (1.5 mos) and median OS (5.5 mos) were shorter for the subgroup of patients with ‘low’ PD-L1 expression (45,46).
M7824
M7824 is a first-in-class bifunctional fusion protein, combining human anti-PD-L1 IgG1 monoclonal antibody with two (2) extracellular domains of TGF-Beta receptor II. This was developed based on basic science research noting that inhibition of TGF-Beta pathway, which promotes tumor immunosuppression. Coupling this with PD-L1 monoclonal antibodies is hoped to enhance the response to PD-L1 inhibition. Two recent phase I studies were presented at ESMO 2018 Congress in advanced esophageal adenocarcinoma after platinum chemotherapy (NCT02517398) and in recurrent gastric or GEJ adenocarcinoma and esophageal squamous cell cancer with no further standard treatment options (NCT02699515) (49-53). These gave signals for efficacy with ORRs in the 20–25% range, DCRs between 30–40%, and with similar rates of grade 3–4 TRAEs as other immunotherapies (20–25%) (49-53). ORRs were similar amongst PD-L1 positive (CPS ≥1) and negative esophageal adenocarcinomas 22.2% and 20%, respectively (49,50). Additional studies in larger cohorts are needed to assess efficacy and safety, but the signal for efficacy in advanced disease gives hope for the future role of M7824 in the treatment of ESCC, as well as esophageal, GEJ, and gastric adenocarcinomas.
Nivolumab
Nivolumab is the second most studied ICI in esophageal, GEJ, and gastric cancers. However, despite several ongoing clinical trials and a promising phase III results out of Japan, it has not gained FDA approval in the US for any of these diseases. The ONO-4538-07 (JapicCTI-No.142422) phase II study investigating the use of Nivolumab in advanced, previously treated ESCC, Adenosquamous carcinoma and adenocarcinoma of the esophagus was published in 2017 (17). The ORR and grade 3–4 TRAEs were both 17%, with mPFS of 1.5 mos and an 11% discontinuation rate due to TRAEs (see Table 1), showing favorable tolerability and a modest signal of efficacy given that 68% of patients had received at least 3 previous chemotherapy regimens (17). Both the ATTRACTION-2 (NCT02267343) and CheckMate-032 (NCT01928394) studies have published results, with ATTRACTION-2 presenting updated results at ESMO 2018 Congress (18-21,54). ATTRACTION-2 is one of the largest published studies in this collection of diseases to date, comparing nivolumab vs. placebo in third-line or later treatment of unresectable, advance, or recurrent GEJ or gastric cancer (18,19,54). Compared with best supportive care and placebo, it prolonged median PFS by 0.2 months (1.61 vs. 1.45 months, P<0.0001) and median OS by 1 month (5.26 vs. 4.14 months, P<0.0001) with good safety profile (grade 3–5 TRAEs: 12% vs. 6%) in these heavily pretreated patients (18-21,54). CheckMate-032 then explored combination immune checkpoint inhibition in a multi-cohort phase I/II study in locally advanced or metastatic adenocarcinomas of the esophagus, GEJ or stomach (20,21). In the second-line or later setting, single agent nivolumab showed only modest efficacy, agnostic of PD-L1 or MSI status. However, responses in PD-L1-positive and MSI-H tumors were more robust, with TRAEs similar to other Nivolumab studies. Further combinations of Nivolumab and Ipilimumab were studied with Nivolumab 1 mg/kg and Ipilimumab 3 mg/kg yielding higher ORR/DCR, again with better responses seen in PD-L1-positive or MSI-H disease; however, this came at the price of doubled toxicity rates and a seven-fold increase in discontinuation rates compared with single agent Nivolumab (20,21). As a result of this early promise in pre-treated disease, several large phase II and III studies are ongoing in the US utilizing in the adjuvant setting, as well as first-line, advanced or metastatic disease with combination immunotherapy, immunochemotherapy or immunochemotherapy with other targeted therapies with study completion dates between December 2021 and October 2024 (70-74,81-83). The studies include the MOONLIGHT, CA224-060, CheckMate-577, CheckMate-648, and CheckMate-649 trials (70-74,81-83).
Pembrolizumab
Pembrolizumab is the most studied ICI in upper GI malignancies, with numerous large studies currently enrolling and scheduled for completion by March 2024. While pembrolizumab was already approved in second-line or later setting as discussed above, several recent updates to phase II and phase III KEYNOTE trials have been presented at major oncology conferences. Additional cohorts from the phase II KEYNOTE-059 (NCT02335411) evaluating Pembrolizumab use in combination with chemotherapy, or as monotherapy, in the first-line setting for advanced gastric/GEJ cancers (27-29). Patients receiving pembrolizumab monotherapy were required to have positive PD-L1 expression, defined as CPS greater than or equal to 1, while the combination therapy arm did not require this, but had 64% of participants with PD-L1 positive tumors (27-29). The combination of cisplatin, 5-FU, and pembrolizumab resulted in a 60% ORR with 4% CR and mPFS of 6.6 months (27-29). ORR for patients receiving pembrolizumab monotherapy was 25.8% with CR of 6.5% and mPFS of 3.3 months (27-29). These studies are discussed in detail below in greater detail (see Table 2). One phase II study (NCT02954536) in HER2+ esophageal, GEJ, or gastric adenocarcinoma combining chemotherapy, trastuzumab and pembrolizumab showed promising results, agnostic of PD-L1 status, and with a tolerable side effect profile (55,56). There remains great hope for an increased role of immunotherapy with Pembrolizumab with several large, ongoing phase III studies (see Table 3), including KEYNOTE-585 (neoadjuvant chemoimmunotherapy gastric and GEJ adenocarcinoma), KEYNOTE-590 (chemoimmunotherapy in untreated, advanced or metastatic EAC, ESCC or GEJ adenocarcinoma), and KEYNOTE-811 (HER2+ metastatic gastric or GEJ adenocarcinoma) (75-79).
Tislelizumab (BGB A317)
Tislelizumab is an investigational anti-PD-1 monoclonal antibody currently being studied in numerous disease states as monotherapy and in combination with other treatments (Table 2). The phase 1 dose escalation/expansion study (NCT02407990) across numerous advanced solid tumors showed early promising results in recurrent/refractory gastric or esophageal cancers (55 patients) (61,62). While the preliminary results by Desai et al. did not show robust ORR (6.4% PR), it did show DCR of 32%, comparable to early studies for ICIs in these previously treated, with the promising absence of any grade 3–5 TRAEs (61,62). At the 2019 ASCO GI Cancers Symposium, Xu et al. presented early safety data from the ESCC cohort (15 Chinese patients) of their phase 2 study (NCT03469557) combining tislelizumab with chemotherapy (cisplatin and 5-FU in this cohort) in the first-line setting (63,64). At least sixty-percent (and up to 80%) of patients with inoperable, locally-advanced, or metastatic ESCC (median age 61 years) experienced grade 3–5 TRAEs, with 26.7% (4 patients) discontinuing therapy due to these adverse effects with a median treatment duration of 108 days (63,64). Despite the high percentage of high-grade adverse effects in this small cohort, many of the adverse effects did not result in treatment discontinuation (vomiting was the most common Efficacy data was not matured at time of the abstract publication (63,64). Beyond the aforementioned phase 2 study, there is an ongoing phase 3 study (NCT03430843) comparing the efficacy and tolerability of second-line tislelizumab against chemotherapy with no results published to date (Table 3) (84,85).
Tremelimumab
Tremelimumab (formerly ticilimumab, CP-675,206) is an anti-CTLA-4 monoclonal antibody without any FDA approvals, but has recently received orphan drug status for mesothelioma. Its role in GEJ and gastric adenocarcinoma is currently under investigation as both monotherapy and in combination with durvalumab (see section on durvalumab above regarding combination immune checkpoint inhibition). However, early results by Kelly et al. of a small sample (12 patients) treated with second-line tremelimumab monotherapy showed a very modest response rate (8% PR) and significant toxicities (Gr. 3–4 TRAEs 50%, with 33% discontinuation rate) (47,48). Median PFS and OS were not calculated due to small sample size (47,48). The combination results were more promising with less toxicities (see Table 2).
Conclusions
The treatment of esophageal, GEJ and gastric cancers has begun to evolve in the era of immunotherapy. While small steps have been made in the treatment paradigm of these diseases, there are many questions left unanswered. The approval of Pembrolizumab in a small subset of patients with: PD-L1-positivity, MSI-H and ddMR deficient tumors after failed, or intolerance to, chemotherapeutic treatment is just the beginning. Despite the successes and promise for an ever-expanding role of immunotherapy, there have also been several key failures. Although it may feel like immunotherapy takes the cliched ‘three steps forward, two steps back’ path to approval in the treatment of upper GI malignancies, these treatments continue to represent progress and hope for what lies ahead. We have a tremendous amount to learn with several large studies in their infancy, we are hopeful that continued progress towards improved therapy with less toxicity is just over the horizon. The use of biomarkers, whether it be MSI, or PD-L1 expression (measured by CPS), in determining the potential for efficacy of immune checkpoint inhibition. Several ongoing studies are investigating other potential biomarkers to aid in improving patient selection to maximize benefit seen with these agents.
Acknowledgments
None.
Footnote
Conflicts of Interest: Khaldoun Almhanna, MD, MPH receives consulting fees from Merck. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- SEER Cancer Stat Facts; Esophageal Cancer Bethesda, MD: National Cancer Institute (NCI); 2019. Available online: https://seer.cancer.gov/statfacts/html/esoph.html
- SEER Cancer Stat Facts: Stomach Cancer Bethesda, MD: National Cancer Institute (NCI); 2019. Available online: https://seer.cancer.gov/statfacts/html/stomach.html
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008;26:1435-42. [Crossref] [PubMed]
- Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009;20:666-73. [Crossref] [PubMed]
- Lorenzen S, Schuster T, Porschen R, et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2009;20:1667-73. [Crossref] [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Albertsson M, Johansson B, Friesland S, et al. Phase II studies on docetaxel alone every third week, or weekly in combination with gemcitabine in patients with primary locally advanced, metastatic, or recurrent esophageal cancer. Med Oncol 2007;24:407-12. [Crossref] [PubMed]
- Ilson DH, Wadleigh RG, Leichman LP, et al. Paclitaxel given by a weekly 1-h infusion in advanced esophageal cancer. Ann Oncol 2007;18:898-902. [Crossref] [PubMed]
- Bouché O, Raoul JL, Bonnetain F, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study--FFCD 9803. J Clin Oncol 2004;22:4319-28. [Crossref] [PubMed]
- Ipilimumab (Yervoy): US Food & Drug Administration; 2011 (updated May 8, 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03695952?term=pembrolizumab&cond=Hepatobiliary+Cancer&rank=1
- Atezolizumab (Tecentriq): US Food & Drug Administration; 2016 (updated May 6, 2019. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761034
- Pembrolizumab (Keytruda): 2014; 2014 (updated April 19, 2019. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process
- Nivolumab (Opdivo): US Food & Drug Administration; 2014 (updated May 2, 2019. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125554
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631-9. [Crossref] [PubMed]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Study of ONO-4538 in Unresectable Advanced or Recurrent Gastric Cancer. Available online: https://ClinicalTrials.gov/show/NCT02267343
- Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol 2018;36:2836-44. [Crossref] [PubMed]
- A Study of Nivolumab by Itself or Nivolumab Combined With Ipilimumab in Patients With Advanced or Metastatic Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT01928394
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Study of Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-3475-028/KEYNOTE-28). Available online: https://ClinicalTrials.gov/show/NCT02054806
- Doi T, Piha-Paul SA, Jalal SI, et al. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol 2018;36:61-7. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RWJ, et al. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 2017;35:4003. [Crossref]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- A Study of Pembrolizumab (MK-3475) in Participants With Recurrent or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma (MK-3475-059/KEYNOTE-059). Available online: https://ClinicalTrials.gov/show/NCT02335411
- Bang YJ, Kang YK, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019;22:828-37. [Crossref] [PubMed]
- Wainberg ZA, Yoon HH, Catenacci DVT, et al. Efficacy and safety of pembrolizumab (pembro) alone or in combination with chemotherapy (chemo) in patients (pts) with advanced gastric or gastroesophageal (G/GEJ) cancer: Long-term follow up from KEYNOTE-059. J Clin Oncol 2019;37:4009. [Crossref]
- Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. [Crossref] [PubMed]
- A Study of Pembrolizumab (MK-3475) Versus Paclitaxel for Participants With Advanced Gastric/Gastroesophageal Junction Adenocarcinoma That Progressed After Therapy With Platinum and Fluoropyrimidine (MK-3475-061/KEYNOTE-061). Available online: https://ClinicalTrials.gov/show/NCT02370498
- Study of Pembrolizumab (MK-3475) in Previously-Treated Participants With Advanced Carcinoma of the Esophagus or Esophagogastric Junction (MK-3475-180/KEYNOTE-180). Available online: https://ClinicalTrials.gov/show/NCT02559687
- Shah MA, Kojima T, Hochhauser D, et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol 2019;5:546-50. [Crossref] [PubMed]
- FDA grants accelerated approval to pembrolizumab for advanced gastric cancer: US Food & Drug Administration; 2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-advanced-gastric-cancer
- Tabernero J, Cutsem EV, Bang YJ, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. J Clin Oncol 2019;37:LBA4007. [Crossref]
- Study of Pembrolizumab (MK-3475) as First-Line Monotherapy and Combination Therapy for Treatment of Advanced Gastric or Gastroesophageal Junction Adenocarcinoma (MK-3475-062/KEYNOTE-062). Available online: https://ClinicalTrials.gov/show/NCT02494583
- Avelumab in Metastatic or Locally Advanced Solid Tumors (JAVELIN Solid Tumor JPN). Available online: https://ClinicalTrials.gov/show/NCT01943461
- Doi T, Iwasa S, Muro K, et al. Avelumab (anti–PD-L1) in Japanese patients with advanced gastric or gastroesophageal junction cancer (GC/GEJC): updated results from the phase 1b JAVELIN Solid Tumor JPN trial. Ann Oncol 2018;29:viii205-70.
- Shen L, Peng Z, Zhang YQ, et al. Camrelizumab combined with capecitabine and oxaliplatin followed by camrelizumab and apatinib as first-line therapy for advanced or metastatic gastric or gastroesophageal junction cancer: Updated results from a multicenter, open label phase II trial. J Clin Oncol 2019;37:4031. [Crossref]
- A Study of SHR-1210 in Combination With Capecitabine + Oxaliplatin or Apatinib in Treatment of Advanced Gastric Cancer. Available online: https://ClinicalTrials.gov/show/NCT03472365
- Zhang B, Qi L, Wang X, et al. Phase 2 study of camrelizumab (anti-PD-1 antibody) combined with apatinib and chemotherapy for the first-line treatment of advanced esophageal squamous cell carcinoma. J Clin Oncol 2019;37:4033. [Crossref]
- SHR-1210 in Combination With Apatinib and Chemotherapy in Patients With Advanced Esophageal Squamous Cell Cancer. Available online: https://ClinicalTrials.gov/show/NCT03603756
- Mamdani H, Schneider BJ, Abushahin LI, et al. Safety and efficacy of durvalumab following multimodality therapy for locally advanced esophageal and GEJ adenocarcinoma: Results from Big Ten Cancer Research Consortium study. J Clin Oncol 2019;37:4058. [Crossref]
- A Study of Durvalumab (MEDI4736) in Esophageal Cancer. Available online: https://ClinicalTrials.gov/show/NCT02639065
- A Study of Ramucirumab (LY3009806) Plus MEDI4736 in Participants With Advanced Gastrointestinal or Thoracic Malignancies. Available online: https://ClinicalTrials.gov/show/NCT02572687
- Bang YJ, Golan T, Lin CC, et al. Ramucirumab (Ram) and durvalumab (Durva) treatment of metastatic non-small cell lung cancer (NSCLC), gastric/gastroesophageal junction (G/GEJ) adenocarcinoma, and hepatocellular carcinoma (HCC) following progression on systemic treatment(s). J Clin Oncol 2019;37:2528. [Crossref]
- A Phase 1b/2 Study of MEDI4736 With Tremelimumab, MEDI4736 or Tremelimumab Monotherapy in Gastric or GEJ Adenocarcinoma. Available online: https://ClinicalTrials.gov/show/NCT02340975
- Kelly RJ, Lee J, Bang YJ, et al. Safety and efficacy of durvalumab in combination with tremelimumab, durvalumab monotherapy, and tremelimumab monotherapy in patients with advanced gastric cancer. J Clin Oncol 2018;36:4031. [Crossref]
- Tan B, Khattak A, Felip E, et al. M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-Beta, in patients with post-platinum esophageal adenocarcinoma (EAC): Preliminary results from a phase 1 cohort. Ann Oncol 2018;29:viii205-70.
- MSB0011359C (M7824) in Metastatic or Locally Advanced Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT02517398
- MSB0011359C (M7824) in Subjects With Metastatic or Locally Advanced Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT02699515
- Bang Y, Doi T, Kondo S, et al. Updated results from a phase 1 trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-Beta, in patients with pretreated recurrent or refractory gastric cancer. Ann Oncol 2018;29:viii205-70.
- Lin C, Doi T, Muro K, et al. Phase 1 study results from an esophageal squamous cell carcinoma (ESCC) cohort treated with M7824 (MSB0011359C), a bifunctional fusion protein targeting transforming growth factor-beta (TGF-Beta) and-PD-L1. Ann Oncol 2018;29:viii205-70.
- Satoh T, Chen L, Kang Y, et al. A phase 3 study of nivolumab (Nivo) in previously treated advanced gastric or gastric esophageal junction (G/GEJ) cancer (ATTRACTION-2): Two-years update data. Ann Oncol 2018;29:viii20570.
- Phase II Trial of Pembrolizumab With Trastuzumab and Chemotherapy in Advanced HER2 Positive Esophagogastric (EG) Cancer. Available online: https://ClinicalTrials.gov/show/NCT02954536
- Janjigian YY, Maron SB, Chou JF, et al. First-line pembrolizumab (P), trastuzumab (T), capecitabine (C) and oxaliplatin (O) in HER2-positive metastatic esophagogastric adenocarcinoma. J Clin Oncol 2019;37:4011. [Crossref]
- Kato K, Kojima T, Hochhauser D, et al. Pembrolizumab in previously treated metastatic esophageal cancer: Longer term follow-up from the phase 2 KEYNOTE-180 Study. J Clin Oncol 2019;37:4032. [Crossref]
- Study of Pembrolizumab (MK-3475) Versus Investigator's Choice Standard Therapy for Participants With Advanced Esophageal/Esophagogastric Junction Carcinoma That Progressed After First-Line Therapy (MK-3475-181/KEYNOTE-181). Available online: https://ClinicalTrials.gov/show/NCT02564263
- Shah MA, Adenis A, Enzinger PC, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: Phase 3 KEYNOTE-181 study. J Clin Oncol 2019;37:4010. [Crossref]
- Kojima T, Muro K, Francois E, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: Phase III KEYNOTE-181 study. J Clin Oncol 2019;37:2. [Crossref]
- Desai J, Millward M, Chao Y, et al. 387P - Preliminary results from subsets of patients (pts) with advanced gastric cancer (GC) and esophageal carcinoma (EC) in a dose-escalation/expansion study of BGB-A317 an anti PD-1 monoclonal antibody (mAb). Ann Oncol 2017;28:v122-41. [Crossref]
- Study of the Safety, Pharmacokinetics and Antitumor Activities of BGB-A317 in Subjects With Advanced Tumors. Available online: https://ClinicalTrials.gov/show/NCT02407990
- BGB A317 in Combination With Chemotherapy as First-Line Treatment in Adults With Inoperable, Locally Advanced or Metastatic Esophageal, Gastric, or Gastroesophageal Junction Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT03469557
- Xu N, Yuan X, Wang B, et al. Tislelizumab in combination with chemotherapy for the treatment of Chinese patients (pts) with esophageal squamous cell carcinoma (ESCC): Results from one cohort of an ongoing phase 2 study. J Clin Oncol 2019;37:14. [Crossref]
- Derks S, Nason KS, Liao X, et al. Epithelial PD-L2 Expression Marks Barrett's Esophagus and Esophageal Adenocarcinoma. Cancer Immunology Research 2015;3:1123-9. [Crossref] [PubMed]
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. [Crossref] [PubMed]
- FDA Approves New Monotherapy Indication for Merck’s KEYTRUDA® (pembrolizumab) (press release). Kenilworth, NJ: Merck, July 31, 2019.
- Phase II Study of Atezolizumab + FLOT vs. FLOT Alone in Patients With Gastric Cancer and GEJ. Available online: https://ClinicalTrials.gov/show/NCT03421288
- Al-Batran S-E, Pauligk C, Hofheinz R, et al. Perioperative atezolizumab in combination with FLOT versus FLOT alone in patients with resectable esophagogastric adenocarcinoma: DANTE, a randomized, open-label phase II trial of the German Gastric Group of the AIO and the SAKK. J Clin Oncol 2019;37:TPS4142. [Crossref]
- Feeney K, Kelly R, Lipton LR, et al. CA224-060: A randomized, open label, phase II trial of relatlimab (anti-LAG-3) and nivolumab with chemotherapy versus nivolumab with chemotherapy as first-line treatment in patients with gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2019;37:TPS4143. [Crossref]
- An Investigational Study of Immunotherapy Combinations With Chemotherapy in Patients With Gastric or Gastroesophageal Junction (GEJ) Cancers. Available online: https://ClinicalTrials.gov/show/NCT03662659
- Modified FOLFOX Plus/Minus Nivolumab and Ipilimumab in Patients With Previously Untreated Advanced or Metastatic Gastric Cancer. Available online: https://ClinicalTrials.gov/show/NCT03647969
- Al-Batran SE, Pauligk C, Goetze TO, et al. Modified FOLFOX versus modified FOLFOX plus nivolumab and ipilimumab in patients with previously untreated advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction: Moonlight, a randomized phase 2 trial of the German Gastric Group of the AIO. J Clin Oncol 2019;37:TPS4144. [Crossref]
- Efficacy Study of Nivolumab Plus Ipilimumab or Nivolumab Plus Chemotherapy Against Chemotherapy in Stomach Cancer or Stomach/Esophagus Junction Cancer. Available online: https://ClinicalTrials.gov/show/NCT02872116
- Pembrolizumab/Placebo Plus Trastuzumab Plus Chemotherapy in Human Epidermal Growth Factor Receptor 2 Positive (HER2+) Advanced Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (MK-3475-811/KEYNOTE-811). Available online: https://ClinicalTrials.gov/show/NCT03615326
- Janjigian YY, Bang Y-J, Fuchs CS, et al. KEYNOTE-811 pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction cancer (mG/GEJC): A double-blind, randomized, placebo-controlled phase 3 study. J Clin Oncol 2019;37:TPS4146. [Crossref]
- Study of Pembrolizumab (MK-3475) Plus Chemotherapy Versus Placebo Plus Chemotherapy in Participants With Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (MK-3475-585/KEYNOTE-585). Available online: https://ClinicalTrials.gov/show/NCT03221426
- Bang J, van Cutsem E, Fuchs CS, et al. Phase 3 KEYNOTE-585 Study of Chemotherapy (Chemo) + Pembrolizumab (Pembro) vs. Chemo + Placebo as Neoadjuvant/Adjuvant Treatment for Patients (Pts) With Gastric or Gastroesophageal Junction Cancer. Ann Oncol 2018;29:viii205-70.
- First-line Esophageal Carcinoma Study With Chemo vs. Chemo Plus Pembrolizumab (MK-3475-590/KEYNOTE-590). https://ClinicalTrials.gov/show/NCT03189719
- Kato K, Shah MA, Enzinger PC, et al. Phase 3 KEYNOTE-590 Study of Chemotherapy + Pembrolizumab Versus Chemotherapy + Placebo as First-Line Therapy for Patients (Pts) With Advanced Esophageal or Esophagogastric Junction Cancer. Ann Oncol 2018;29:viii205-70.
- An Investigational Immuno-therapy Study of Nivolumab or Placebo in Patients With Resected Esophageal or Gastroesophageal Junction Cancer. Available online: https://ClinicalTrials.gov/show/NCT02743494
- Ajani JA, Kato K, Doki Y, et al. CheckMate 648: A randomized phase 3 study of nivolumab plus ipilimumab or nivolumab combined with fluorouracil plus cisplatin versus fluorouracil plus cisplatin in patients with unresectable advanced, recurrent, or metastatic previously untreated esophageal squamous cell carcinoma. J Clin Oncol 2018;36:TPS193. [Crossref]
- A Study to Evaluate Efficacy in Subjects With Esophageal Cancer Treated With Nivolumab and Ipilimumab or Nivolumab Combined With Fluorouracil Plus Cisplatin Versus Fluorouracil Plus Cisplatin. Available online: https://ClinicalTrials.gov/show/NCT03143153
- A Study of Tislelizumab (BGB-A317) Versus Chemotherapy as Second Line Treatment in Patients With Advanced Esophageal Squamous Cell Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT03430843
- Shen L, Ajani JA, Kim SB, et al. A Phase 3, Randomized, Open-Label Study to Compare the Efficacy of Tislelizumab Versus Chemotherapy as Second-Line Therapy for Advanced Unresectable Metastatic Esophageal Squamous Cell Carcinoma. Ann Oncol 2018;29:viii205-70.
Cite this article as: Zayac A, Almhanna K. Esophageal, gastric cancer and immunotherapy: small steps in the right direction? Transl Gastroenterol Hepatol 2020;5:9.


