
Acute on chronic liver failure in non-alcoholic fatty liver and alcohol associated liver disease
Introduction
Acute on chronic liver failure (ACLF) is a unique clinical syndrome in patients with chronic liver disease and cirrhosis, presenting with acute deterioration in hepatic function that progresses to multiple organ failure, and is associated with a high short-term mortality. The other characteristic feature of ACLF is the potential reversibility of the event with the possibility that patients return to baseline liver function. In contrast, decompensation of cirrhosis that leads to multiple organ failure may not be reversible. This syndrome has been variably defined across the globe, but the common features are acute presentation and high short-term mortality. The precipitating events and the underlying etiology of liver disease however vary across different countries and populations. In this review we discuss the syndrome, definitions across the globe, healthcare burden and epidemiology, pathogenesis, organ failures and treatment specifically in relation to nonalcoholic fatty liver disease (NAFLD) and alcohol associated liver disease (AALD).
Variations on defining ACLF
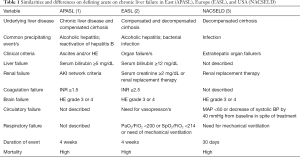
ACLF has been defined differently by various organizations including Asia Pacific (APASL), European and North American consortia, and the World Gastroenterology Organization (1-3). These definitions differ in terms of the duration of acute event, precipitating events, presenting features, underlying liver disease, and definitions of organ failure (Table 1). In a retrospective study of 80,383 veterans with cirrhosis, incidence of ACLF was 5.7 per 1,000 person years as defined by APASL and 20.1 by EASL criteria. The 28 and 90 days mortality for the two groups was 42% and 56% for ACLF patients as per APASL definition, 38% and 50% as per EASL criteria (4). This important study clearly shows that although the rate of ACLF is different depending on which definition is used, the outcome of these patients over a period of 1–3 months remains dismal. The World Gastroenterology Organization attempted to come up with a uniform definition of ACLF as acute deterioration of underlying chronic liver disease with or without cirrhosis leading to multiple organ failure and potential for high mortality (5). It is important to reach consensus on a definition of ACLF that can be used across studies to determine the natural history, pathogenesis, and risk factors, as basis to facilitate development of prognostic models, biomarkers, and therapeutic targets.
Full table
Epidemiology and health care burden
Among hospitalized patients with cirrhosis, the prevalence of ACLF is reported to vary between 5% to 30% (2,3,6-8). The prevalence varies for many reasons across studies due to variations in the definition of ACLF and the type of study (4). For example, in prospective and retrospective single or multicenter studies, the prevalence is reported to be higher as the cases can be characterized more definitively (2,3), compared to database studies where the cases are characterized based on the International Classification of Disease (ICD) codes in the absence of available laboratory values to accurately define organ failures (7,8). In two different database studies on hospitalized patients of cirrhosis in the US, the prevalence of ACLF was reported to be 5% in 2011 and 6.6% in 2014 (8,9). The prevalence of ACLF has increased over time from as low as 1.5% among patients with cirrhosis admitted to various hospitals in the US in 2001 (7). This is probably related to wider recognition of the syndrome but may also be related to relatively better care with patients living longer while they are on the transplant list. Although any liver disease etiology can be associated with ACLF, the increasing prevalence of obesity has probably resulted in non-alcoholic steatohepatitis (NASH) emerging as the most rapidly growing etiology of liver disease associated with ACLF among hospitalized patients with cirrhosis (8). In another study, morbid obesity was recognized as an important risk factor for the development of ACLF in patients with liver cirrhosis (10). ACLF can occur in both compensated and decompensated cirrhosis. It can also occur, and has been reported in patients with chronic liver disease without cirrhosis. For example, a patient with chronic hepatitis C virus infection or NAFLD or NASH without cirrhosis may present with ACLF after drug induced liver injury, or post-surgery decompensation. Similarly, many patients with Wilson’s disease, alcoholic hepatitis, and autoimmune hepatitis do not have underlying cirrhosis but have pre-existent chronic liver disease when they present with ACLF.
Cirrhosis is the 8th leading cause of death contributing to mortality rate of 25.7 per 100,000 general population and 40,000 deaths per year in the US. Cirrhosis also leads to substantial morbidity with over 250,000 hospitalizations in 2014 and estimated cost of approximately 4 billion US dollars (11-14). A major component of this healthcare burden is contributed by patients with ACLF, as the development of this syndrome is associated with in-hospital mortality rate of 44% (8). Of all the liver disease etiologies, hepatitis C virus related ACLF has the highest in-hospital mortality at 54% followed by AALD, other causes of liver disease, and least in NASH patients at 34% (8). The ACLF associated in-hospital mortality is decreasing from over 65% in 2001 to 50% in 2011 and less than 40% in 2014, probably due to better patient care and more awareness of the syndrome among providers with early recognition (8). However, the healthcare burden and economic burden associated with this illness continues to increase. For example, the inpatient cost of hospitalized patients with ACLF is 3–4-fold higher as compared to cost incurred on patients hospitalized with cirrhosis not complicated by ACLF. Further, this cost has increased over time from US$320 million in 2001 to 1.7 billion USD in 2011 (9).
Pathogenesis of ACLF
Precipitants
The most common precipitant is bacterial infection which sets the stage for acute hepatic decompensation manifesting as ACLF (2,3). Cirrhosis is a risk factor for infections with prevalence of 30% among hospitalized patients compared to 5–7% among hospitalized patients without cirrhosis (15,16). The immune compromised state in cirrhosis is multifactorial including but not limited to low levels of complement and serum albumin, malnutrition, reduced phagocytic activity of neutrophils, lymphopenia, and reduced ability of monocytes to present foreign antigens to the immune system (15,17). It may often be difficult to decipher whether infection is really a precipitant or complication of ACLF as patients with liver failure and ACLF are at risk for infections (18). In Asia, reactivation of hepatitis B virus infection is a common event precipitating ACLF (1). Other common precipitants are gastrointestinal bleeding, drug induced liver injury, surgical or physical trauma, and acute portal vein thrombosis (19,20). Among patients with AALD, apart from these precipitants, heavy alcohol use precipitates ACLF, a condition peculiar to AALD patients and termed as alcohol associated hepatitis (AAH) (21,22). In about 30–40% cases, the precipitant remains unknown, and it is possible that many of these cases may be precipitated by infection which remains undiagnosed (1-3). Specific to NASH, the mechanisms and pathogenesis of obesity with increased predisposition to ACLF development in patients with chronic liver disease remain unclear and need to be addressed in future studies.
Mechanisms of ACLF
Systemic inflammation
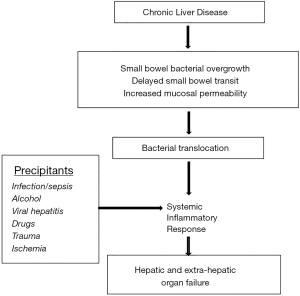
Systemic inflammation is the signature mechanism of ACLF, and is associated with cytokine response (Figure 1). The evidence in favor of systemic inflammation in ACLF stems from many studies that show C-reactive protein, systemic inflammatory response syndrome (SIRS), lipopolysaccharide and bacterial DNA, and neutrophil-lymphocyte ration to be associated with ACLF severity and outcomes (18,23-26). Inflammation can be sterile or related to infection. For example, in AAH patients translocation of bacterial endotoxin and lipopolysaccharide across the portal circulation results in activation of toll like-4 (TLR-4) or pattern associated recognition molecular (PAMP) receptors on the hepatic macrophages (27). This leads to induction of cytokine response via interleukin-1 (IL-1) and caspase-1 mediated inflammasome formation. Microbiome changes with bacterial overgrowth and dysbiosis, increased intestinal permeability, reduced bactericidal activity of innate immune cells in the gastrointestinal mucosa, and abnormalities in tight junctions of the enterocytes result in translocation of bacterial DNA and lipopolysaccharide into the portal circulation and the liver parenchyma (18,25,28). Hepatic inflammation induced by any precipitant leads to cell death releasing cellular material or danger associated molecular patterns (DAMP), which in turn can activate TLR-4 receptors on the hepatic macrophages perpetuating the hepatic inflammation, cytokine response, and systemic inflammation (27,28). The inflammatory cascade is associated with secretion of extra-cellular vesicles or EVs which further mediate recruitment of inflammatory cells perpetuating the systemic and hepatic inflammation. These EVs recently have been shown to contain specific mRNAs. The density of EVs and specific mRNA characterization has been associated with prediction and worse outcome in AAH patients.
AAH is characterized by activation of pro- and anti-inflammatory pathways activation, the latter mediating hepatic regeneration and proliferation, but also immune paralysis. The pro-inflammatory pathways (SIRS) are activated by IL-1 family mainly IL-8 in AAH and IL-6 in infection induced ACLF. The anti-inflammatory pathways or compensatory anti-inflammatory response syndrome (CARS) are activated by IL-10 family including IL-22 and hepatic growth factor (29). Neutrophils recruited from the bone marrow are involved in mediating hepatic regeneration, and this is evidenced by the density of neutrophils on liver biopsy being associated with better outcome in AAH patients (30). However, the cytokine storm in severe ACLF is associated with more prominent CARS activity leading to exhaustion of immune system with its consequent paralysis. This stage is associated with reduced HLA-DR activity on monocytes, increased expression of inhibitory molecule tyrosine-protein kinase Mer (MERTK receptor) and its ligands (31,32).
Microcirculatory changes
Microcirculatory changes within the hepatic and splanchnic vasculature are associated with inflammation and cytokine signaling. These include decreased activity of endothelial nitric oxide synthase (eNOS) activity in the hepatic and increased activity in splanchnic circulation, along with increased activity of endothelin-1 and thromboxane in the hepatic microvasculature (33,34). These changes lead to sinusoidal portal hypertension, with worsening of splanchnic pooling and reduction in effective circulatory blood volume. Hepatocytes, hepatic macrophages, recruited inflammatory cells, endothelial cellular activation all interact with hepatic stellate cells resulting in vasoconstriction, hepatic fibrosis and further worsening of portal hypertension (Figure 1) (29).
Diagnosis of ACLF
Criteria for the diagnosis of ACLF are different depending on which definition of ACLF is used. Of all the organ failures, brain failure is consistent across these definitions with grade III or IV encephalopathy (Table 1). The number of organ failures determines the severity of ACLF. For example, using the EASL definition, two organ failures or one extra-renal failure with serum creatinine 1.5–1.9 mg/dL can diagnose ACLF-1, 3 organ failures is consistent with ACLF-2, and >3 organ failures diagnoses most severe or ACLF-3 (2). Using the NACSELD definition, presence of 2, 3, or 4 extrahepatic organ failures define ACLF-1, ACLF-2, and ACLF-3 respectively (3). However, APASL criteria do not define and grade the severity of ACLF.
Treatment of ACLF
Patients with ACLF who are critically sick often require intensive care with criteria and level of care similar to patients without chronic liver disease/cirrhosis (35).
Management of organ failures
Renal failure
Renal failure is the most common organ failure in ACLF patients. Early diagnosis and treatment of acute kidney injury (AKI) is important to improve outcome of these patients (36). If the renal function does not improve with volume replacement and correction of pre-renal reversible factors, vasoconstrictor therapy such as with terlipressin combined with intra-venous albumin is the next step if the diagnosis is hepatorenal syndrome. Renal replacement therapy is usually offered to patients with HRS listed or considered for liver transplantation (LT), and if AKI is secondary to acute tubular necrosis which may occur with sepsis (37).
Brain failure
Brain failure or grade III–IV hepatic encephalopathy is treated in the same way as hepatic encephalopathy in decompensated cirrhosis, with use of lactulose with or without rifaximin. Endotracheal intubation may often be needed for airway protection. Patients with atypical features such as sudden onset of mental status change, seizures, focal deficit, and meningeal signs should warrant search for alternative diagnosis. In such patients, brain imaging, electroencephalogram, or lumbar puncture examination may be required. For patients’ refractory to treatment, diagnosis of hepatic encephalopathy needs reconsideration and continuing efforts to uncover precipitants. Contrast enhanced abdominal CT or MR scan may be performed to look for spontaneous portosystemic shunts and if found, closure of these shunts may be required (38).
Respiratory failure
Respiratory failure may be multifactorial, from fluid overload, acute respiratory distress syndrome, pneumonia, atelectasis, or pleural effusion. If mechanical ventilation is needed, it is recommend that low tidal volume with lung protective strategies be used (39).
Circulatory failure
Hyper dynamic state with reduced effective circulatory blood volume in patients with cirrhosis predisposes these patients to hypotension compromising tissue perfusion. This can be complicated by structural changes and myocardial dysfunction (cirrhotic cardiomyopathy) in cirrhosis patients (40). Transthoracic echocardiography and use of central venous pressure monitoring, pulmonary artery catheterization, and/or arterial line placement may be needed to help the management of these patients (20,37). Treatment measures include volume expansion with albumin and/or saline, treatment of associated infections/sepsis with appropriate antibiotics, and use of vasopressors.
Coagulation failure
No specific interventions are needed except that blood products may be needed for active bleeding and prior to invasive procedures. It is recommended that the serum fibrinogen level be kept above 100–120 mg/dL in patients who are bleeding. Rotational thromboelastography or ROTEM may be performed to guide treatment and has been shown to reduce the need for blood products and substitution of coagulation factors (41).
Management of infections
Infection is a common precipitant as well as complication of ACLF, and is the main focus of ACLF treatment (16,20,37). Comprehensive infectious work-up is recommended including blood and urine cultures, ascitic fluid examination for spontaneous bacterial peritonitis, detailed history and physical examination for skin and subcutaneous source of infection, and chest X-ray. Pending culture results, antibiotic administration is important as delay in administration of antibiotics is negatively associated with outcome. For patients suspected to have healthcare associated (prior hospitalization within last 30 days or coming for nursing home) or nosocomial (infections occurring after 48 hours of hospitalization) infection, broader coverage for gram negative (cefepime, piperacillin tazobactam, or meropenem) and gram positive (vancomycin or linezolid) organisms is required, as these infections are often associated with multi-drug resistant (MDR) organisms (42). With increasing use of antibiotics, the prevalence of MDR (almost 34% and higher in Asian countries) and fungal infections is increasing (42-44). This is further complicated with use of certain medications including corticosteroids for AAH (43,44). The outcome of infections with MDR organisms is poorer with suboptimal response to antibiotics and higher odds for septic shock (42). For patients with watery diarrhea, stool for Clostridium difficile toxin should be checked (16,45).
Liver support devices have not demonstrated survival benefit and are not recommended outside of trials.
Specific pharmacological management
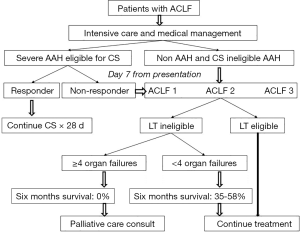
Currently, no specific pharmacological treatment exists for ACLF patients except for AAH patients. Corticosteroids (prednisolone 40 mg orally or 32 mg intravenous methylprednisolone) is recommended (Figure 2) for patients with severe episode (Maddrey’s discrimination function ≥32 or MELD >20) eligible to receive corticosteroids (46,47). About 50–60% severe AAH patients respond to corticosteroids which is assessed at 4–7 days of treatment by the Lille score, and these patients continue treatment for another 3 weeks, whereas treatment is discontinued in non-responders (48). Corticosteroids improve one month survival in patients with AAH, but do not impact the long term outcome (49-51).
Liver transplantation
LT has traditionally been contraindicated in AAH as transplant centers all over the world traditionally required at least six months of sobriety before considering LT. However, psychosocial support, young age, and other criteria are probably more important than minimum six months of sobriety in predicting relapse to alcohol use after LT (52). Over the last 5–10 years, many studies have shown survival benefit with early LT in AAH patients (53-55), and many transplant centers now do not mandate six months sobriety for considering LT for AAH patients, and consider this therapeutic for select AAH patients (Figure 2) (46).
However, criteria for LT in management of ACLF patients outside AAH setting are evolving. In few small studies, patients receiving LT for select ACLF patients have had excellent outcomes (56-58). In a recent study, patients with ACLF-3 had higher waitlist mortality or risk of delisting within 28 days compared to patients with lower ACLF grades or without ACLF. Further, within ACLF-3, this risk was highest at 44% for patients with listing MELD score <29 compared to those with higher MELD scores (58). LT within 30 days of listing among ACLF-3 patients was associated with improved survival. Among patients selected for LT, mechanical ventilation at transplant and use of poor quality graft (donor risk index >1.70) were negatively associated with one year post-transplant survival (58).
Palliative care
Many patients with ACLF do not respond to treatment and are not candidates for LT. It is appropriate to initiate a discussion on goals of care for these patients. These patients not only consume healthcare resources, but also suffer symptoms that negatively impact on their quality of life (8,9,59). In one study, 48% of patients who were delisted from LT list remained in the ICU with median time to death of 52 days. On Edmonton symptom assessment scale, these patients had a plethora of symptoms including pain (65%), nausea (58%), 46% (anxiety or depression), shortness of breath (48%), and anorexia (49%) (59). The use of palliative care is very infrequent in ACLF patients, with only 5–11% consulted for palliative care and only 29% had a documented ‘do not resuscitate’ status (8,59,60). Patients with ACLF-3 with ≥4 organ failures or with CLIF-organ failure score of ≥70 at 7 days from presentation who are in the intensive care unit and are not LT candidates have 100% 90-d mortality (Figure 2) (61,62). It is hence appropriate to consider discussion of goals of care in these patients early on, with involvement of palliative care specialty to optimize their management and reduce use of healthcare resources (61,62).
Prognosis
ACLF has high short-term mortality. The mortality is directly related to the number of organ failures or severity/grade of ACLF with 22%, 33%, and 70% 28-d mortality for ACLF-1, ACLF-2, and ACLF-3 respectively. Similar figures for 90-d mortality are 40%, 55%, and 75% respectively (2). Similarly, in-hospital mortality of all hospitalized patients with ACLF is 44% and increases with grade of ACLF (8). Based on a prospective study, the chronic liver failure-sequential organ failure assessment (CLIF-SOFA) score has been developed for ACLF patients, with a score of ≥70 after 48 hours of stay in the intensive care unit is associated with 100% mortality at 28-d (62). Model for End-stage Liver Disease (MELD) score, Acute Physiology and Chronic Health Evaluation (APACHE), and North American Consortium for End Stage Liver Disease (NACSELD) are other available scoring systems (20). However, none of these is an ideal scoring system (58). Healthcare or nosocomial vs. community acquired and second vs. first infections are associated with worse outcomes (16,42,63). Blood ammonia levels in patients with ACLF and in patients with AAH has been shown to be negatively associated with patient survival (64,65). In AAH patients, liver histology can also predict severity and outcomes using histologic scoring system based on degree of neutrophil infiltration, ductular or canalicular cholestasis, cirrhosis, and presence of mega mitochondria (30).
Summary and future prospects
ACLF is a recently recognized clinical syndrome with high short-term mortality. Although, the criteria to characterize ACLF patients are well described, much remains to be done in this developing field including but not limited to homogenizing the definition across the globe, describing true epidemiology and healthcare burden from ACLF, identifying biomarkers for early recognition of ACLF and of organ dysfunction, describing natural history of ACLF, determining accurate prognostic scores, homogenizing criteria for patient selection for LT, and early identification of patients who require palliative care. Further, studies are needed to describe characteristics and epidemiology specific to liver disease etiology related ACLF.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009;3:269-82. [Crossref] [PubMed]
- Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426-37, 37.e1-9.
- Bajaj JS, O'Leary JG, Reddy KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250-6. [Crossref] [PubMed]
- Mahmud N, Kaplan DE, Taddei TH, et al. Incidence and Mortality of Acute-on-Chronic Liver Failure Using Two Definitions in Patients with Compensated Cirrhosis. Hepatology 2019;69:2150-63. [Crossref] [PubMed]
- Jalan R, Yurdaydin C, Bajaj JS, et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology 2014;147:4-10. [Crossref] [PubMed]
- O'Leary JG, Reddy KR, Garcia-Tsao G, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology 2018;67:2367-74. [Crossref] [PubMed]
- Allen AM, Kim WR. Epidemiology and Healthcare Burden of Acute-on-Chronic Liver Failure. Semin Liver Dis 2016;36:123-6. [Crossref] [PubMed]
- Axley P, Ahmed Z, Arora S, et al. NASH Is the Most Rapidly Growing Etiology for Acute-on-Chronic Liver Failure-Related Hospitalization and Disease Burden in the United States: A Population-Based Study. Liver Transpl 2019;25:695-705. [Crossref] [PubMed]
- Allen AM, Kim WR, Moriarty JP, et al. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology 2016;64:2165-72. [Crossref] [PubMed]
- Sundaram V, Jalan R, Ahn JC, et al. Class III obesity is a risk factor for the development of acute-on-chronic liver failure in patients with decompensated cirrhosis. J Hepatol 2018;69:617-25. [Crossref] [PubMed]
- Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591-608. [Crossref] [PubMed]
- Scaglione S, Kliethermes S, Cao G, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol 2015;49:690-6. [Crossref] [PubMed]
- Kim WR, Brown RS Jr, Terrault NA, et al. Burden of liver disease in the United States: summary of a workshop. Hepatology 2002;36:227-42. [Crossref] [PubMed]
- Asrani SK, Larson JJ, Yawn B, et al. Underestimation of liver-related mortality in the United States. Gastroenterology 2013;145:375-82.e1-2.
- Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:727-38. [Crossref] [PubMed]
- Singal AK, Salameh H, Kamath PS. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalised patients in the United States. Aliment Pharmacol Ther 2014;40:105-12. [Crossref] [PubMed]
- Singal AK, Charlton MR. Nutrition in alcoholic liver disease. Clin Liver Dis 2012;16:805-26. [Crossref] [PubMed]
- Singal AK, Shah VH, Kamath PS. Infection in Severe Alcoholic Hepatitis: Yet Another Piece in the Puzzle. Gastroenterology 2017;152:938-40. [Crossref] [PubMed]
- Asrani SK, Simonetto DA, Kamath PS. Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol 2015;13:2128-39. [Crossref] [PubMed]
- Bajaj JS, Moreau R, Kamath PS, et al. Acute-on-Chronic Liver Failure: Getting Ready for Prime Time? Hepatology 2018;68:1621-32. [Crossref] [PubMed]
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009;360:2758-69. [Crossref] [PubMed]
- Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014;12:555-64; quiz e31-2.
- Piano S, Morando F, Carretta G, et al. Predictors of Early Readmission in Patients With Cirrhosis After the Resolution of Bacterial Infections. Am J Gastroenterol 2017;112:1575-83. [Crossref] [PubMed]
- Michelena J, Altamirano J, Abraldes JG, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015;62:762-72. [Crossref] [PubMed]
- Vergis N, Atkinson SR, Knapp S, et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology 2017;152:1068-77.e4. [Crossref] [PubMed]
- Vergis N, Khamri W, Beale K, et al. Defective monocyte oxidative burst predicts infection in alcoholic hepatitis and is associated with reduced expression of NADPH oxidase. Gut 2017;66:519-29. [Crossref] [PubMed]
- Singal AK, Louvet A, Shah VH, et al. Grand Rounds: Alcoholic Hepatitis. J Hepatol 2018;69:534-43. [Crossref] [PubMed]
- Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015;148:30-6. [Crossref] [PubMed]
- Singal AK, Kodali S, Vucovich LA, et al. Diagnosis and Treatment of Alcoholic Hepatitis: A Systematic Review. Alcohol Clin Exp Res 2016;40:1390-402. [Crossref] [PubMed]
- Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014;146:1231-9.e1-6.
- Markwick LJ, Riva A, Ryan JM, et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology 2015;148:590-602.e10. [Crossref] [PubMed]
- Bernsmeier C, Pop OT, Singanayagam A, et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology 2015;148:603-15.e14. [Crossref] [PubMed]
- Gronbaek H, Sandahl TD, Mortensen C, et al. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment Pharmacol Ther 2012;36:173-80. [Crossref] [PubMed]
- Laleman W, Landeghem L, Wilmer A, et al. Portal hypertension: from pathophysiology to clinical practice. Liver Int 2005;25:1079-90. [Crossref] [PubMed]
- Meersseman P, Langouche L, du Plessis J, et al. The intensive care unit course and outcome in acute-on-chronic liver failure are comparable to other populations. J Hepatol 2018;69:803-9. [Crossref] [PubMed]
- Angeli P, Rodriguez E, Piano S, et al. Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut 2015;64:1616-22. [Crossref] [PubMed]
- Jalan R, Moreau R, Kamath PS, et al. Acute-on-Chronic Liver Failure: A Distinct Clinical Condition. Semin Liver Dis 2016;36:107-8. [Crossref] [PubMed]
- Laleman W, Simon-Talero M, Maleux G, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology 2013;57:2448-57. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Moller S, Hove JD, Dixen U, et al. New insights into cirrhotic cardiomyopathy. Int J Cardiol 2013;167:1101-8. [Crossref] [PubMed]
- Bedreli S, Sowa JP, Gerken G, et al. Management of acute-on-chronic liver failure: rotational thromboelastometry may reduce substitution of coagulation factors in liver cirrhosis. Gut 2016;65:357-8. [Crossref] [PubMed]
- Piano S, Singh V, Caraceni P, et al. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology 2019;156:1368-80.e10. [Crossref] [PubMed]
- Gustot T, Maillart E, Bocci M, et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J Hepatol 2014;60:267-74. [Crossref] [PubMed]
- Hmoud BS, Patel K, Bataller R, et al. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: a meta-analysis of randomized trials. Liver Int 2016;36:721-8. [Crossref] [PubMed]
- Bajaj JS, Ananthakrishnan AN, Hafeezullah M, et al. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. Am J Gastroenterol 2010;105:106-13. [Crossref] [PubMed]
- Singal AK, Bataller R, Ahn J, et al. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol 2018;113:175-94. [Crossref] [PubMed]
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012;57:399-420. [Crossref] [PubMed]
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348-54. [Crossref] [PubMed]
- Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619-28. [Crossref] [PubMed]
- Singh S, Murad MH, Chandar AK, et al. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology 2015;149:958-70.e12. [Crossref] [PubMed]
- Louvet A, Thursz MR, Kim DJ, et al. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology 2018;155:458-68.e8. [Crossref] [PubMed]
- McCallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol Alcohol 2006;41:358-63. [Crossref] [PubMed]
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790-800. [Crossref] [PubMed]
- Singal AK, Bashar H, Anand BS, et al. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012;55:1398-405. [Crossref] [PubMed]
- Marot A, Dubois M, Trepo E, et al. Liver transplantation for alcoholic hepatitis: A systematic review with meta-analysis. PLoS One 2018;13:e0190823. [Crossref] [PubMed]
- Gustot T, Agarwal B. Selected patients with acute-on-chronic liver failure grade 3 are not too sick to be considered for liver transplantation. J Hepatol 2017;67:667-8. [Crossref] [PubMed]
- Artru F, Louvet A, Ruiz I, et al. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol 2017;67:708-15. [Crossref] [PubMed]
- Sundaram V, Jalan R, Wu T, et al. Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology 2019;156:1381-91.e3. [Crossref] [PubMed]
- Poonja Z, Brisebois A, van Zanten SV, et al. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol 2014;12:692-8. [Crossref] [PubMed]
- Tandon P, Reddy KR, O'Leary JG, et al. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology 2017;65:217-24. [Crossref] [PubMed]
- Gustot T, Fernandez J, Garcia E, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243-52. [Crossref] [PubMed]
- Engelmann C, Thomsen KL, Zakeri N, et al. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care 2018;22:254. [Crossref] [PubMed]
- Bajaj JS, O'Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology 2012;56:2328-35. [Crossref] [PubMed]
- Shalimar, Sheikh MF, Mookerjee RP, et al. Prognostic Role of Ammonia in Patients With Cirrhosis. Hepatology 2019;70:982-94.
- Ravi S, Bade KS, Hasanin M, et al. Ammonia level at admission predicts in-hospital mortality for patients with alcoholic hepatitis. Gastroenterol Rep (Oxf) 2017;5:232-6. [PubMed]
Cite this article as: Singal AK, Kamath PS. Acute on chronic liver failure in non-alcoholic fatty liver and alcohol associated liver disease. Transl Gastroenterol Hepatol 2019;4:74.


