Role of laparoscopic cholecystectomy in the management of chronic right upper quadrant pain due to biliary dyskinesia: a systematic review and meta-analysis
Introduction
Biliary dyskinesia is a gallbladder motility disorder associated in majority of patients with right upper quadrant abdominal pain (1,2). This pain mimics biliary colic except the patients do not have gallstones on abdominal ultrasonography or magnetic resonance cholangiopancreatography, or other conditions that could usually cause pain in this area such as peptic ulcer disease, pancreatitis and colonic diverticular disease (2). The cholescintigraphy has been used to aid in the diagnosis of biliary dyskinesia (3,4), favouring it when gallbladder ejection fraction is low (traditionally <35%) (5). Biliary dyskinesia is a less clearly defined term (6). The ambiguity in diagnosing this condition, a lack of consistency in its management, and the background of its significant presence in the community (up to 21% in females); all these make it an issue that burdens both economy and health-care resources (7). Laparoscopic cholecystectomy (LC) has been performed for biliary dyskinesia with varying degrees of success. Several studies have reported significant improvement of symptoms in patients undergoing cholecystectomy than those who did not (8-10). This review is an attempt to answer several questions related to the surgical management of biliary dyskinesia.
The objective of this study is to evaluate the surgical outcomes and feasibility of performing LC in patients with longstanding right upper quadrant pain due to biliary dyskinesia.
Methods
Data sources and search strategy
A search of customary medical electronic databases such as MEDLINE, EMBASE, and Cochrane Library for randomized, controlled trials and other comparative studies was conducted. The medical subject headings (MeSH) search terms reported in the Medline library relevant to the target subject were used to select relevant studies. These included “acalculous biliary pain”, “biliary dyskinesia”, “cholescintigraphy”, “functional gallbladder disorders”, “laparoscopic cholecystectomy” and “right upper quadrant pain”. The limitations for language, gender, age, sample size and place of study origin were removed from the search engine. Boolean operators (AND, OR, NOT) were used to narrow and widen the resulting outcomes of search results. The published titles from the search results were screened appropriately and their inclusion or exclusion was determined according to the predefined criteria. In addition, the reference list from the selected articles was also scrutinized as a further search tool to find additional trials.
Study selection
For inclusion in the meta-analysis, a study had to meet the following criteria: (I) a randomised, or non-randomised, controlled trial; (II) comparison between LC and non-cholecystectomy group (NLC); (III) the reported follow-up to evaluate the resolution of symptoms following intervention. All trials, case reports, reviews and abstracts with inadequate data or not meeting the above-mentioned inclusion criteria were excluded from the study.
Data extraction
Two independent reviewers (S Rehman and MS Sajid), using a predefined meta-analysis form, extracted data from each study which resulted in a high and satisfactory inter-observer agreement. The documented variables in the pre-defined meta-analysis profroma were the name of the authors, the title of the study, the journal in which the study was published, the country and year of the study, intervention regimen, no-intervention regimen, the length of the therapy, testing sample size (with sex differentiation if applicable), the number of patients receiving each regimen within the group, the number of patients who succeeded and failed the allocated treatment, the patient compliance rate in each group, the number of patients reporting complications and the number of patients with absence of complications in each arm. Third reviewer (KK Singh) confirmed the data and all three reviewers discussed the results and, if discrepancies were present, a consensus was reached.
Data synthesis and statistical analysis
The software package RevMan 5.3 (11,12), provided by the Cochrane Collaboration, was used for the statistical analysis to achieve a combined outcome. The risk ratio (RR) with a 95 per cent confidence interval (CI) was calculated for binary data, and the standardised mean difference (SMD) with a 95 per cent CI was calculated in case of continuous data variables. The random-effects model (13-15) was used to calculate the outcomes of variables. Heterogeneity was explored using the chi2 test, with significance set at P<0.05, and was quantified (16) using I2, with a maximum value of 30 per cent identifying low heterogeneity, up to 66% suggesting moderate heterogeneity and more than 66% suggesting significant heterogeneity (17). The Mantel-Haenszel method was used for the calculation of RR under the random effect models (18). In a sensitivity analysis, 0.5 was added to each cell frequency for trials in which no event occurred in either the treatment or control group, according to the method recommended by Deeks et al. (18). If the standard deviation was not available then it was calculated according to the guidelines of the Cochrane Collaboration (19). This process involved assumptions that both groups had the same variance, which may not have been true, and variance was either estimated from the range or from the p-value. The estimate of the difference between both techniques was pooled, depending upon the effect weights in results, determined by each trial estimated variance. A forest plot was used for the graphical display of the results. The square around the estimate stood for the accuracy of the estimation (sample size), and the horizontal line represented the 95% CI. The methodological quality of the included randomised trials was initially assessed using the published guidelines of Jadad et al. and Chalmers et al. (20,21). Based on the quality of the included trials, the strength and summary of the evidence was further evaluated by GradePro® (22), a tool provided by the Cochrane Collaboration.
Endpoints
Complete resolution of symptoms was analysed as the primary endpoint in this study. Secondary endpoints included partial resolution and no-resolution of symptoms.
Results
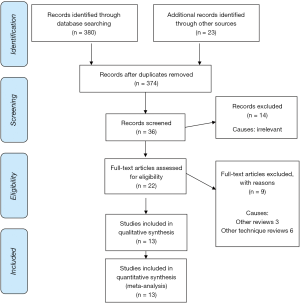
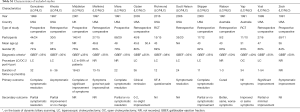
The PRISMA flow chart to explain the literature search strategy and trial selection is given in Figure 1. Thirteen studies (two randomised, controlled trials and 11 comparative studies) (23-35) recruiting 740 patients were retrieved from the search of medical electronic databases. There were 542 patients in the LC group and 198 patients in the NLC group. The characteristics of the included trials are given in Table S1.
Full table
Methodological quality of included studies
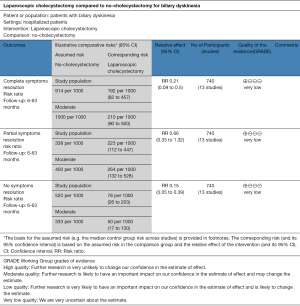
According to Jadad et al. and Chalmers et al. (17,18), the quality of two included randomised trials was good due to the satisfactory utilization of randomization techniques. In addition, there was adequate reporting of power calculation, allocation concealment and intention-to-treat analysis. The quality of retrospective and prospective comparative trials was moderate based upon their review as per Scottish Intercollegiate Guidelines Network (SIGN). Based on the quality of included studies, the strength and summary of evidence analysed on GradePro® (22) is given in Figure 2 which is expected to be of low quality due to paucity of high powered randomised, controlled trials.
Complete resolution of symptoms
There was significant heterogeneity (Tau2 =1.99, Chi2 =201.97, df =11, P<0.00001: I2 =95%) among trials. In the random effects model analysis (RR, 0.21; 95% CI, 0.09, 0.50; z =3.49; P=0.0005. Figure 3), complete resolution was more likely in LC group compared to NLC group.
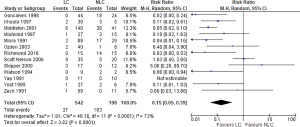
Partial resolution of symptoms
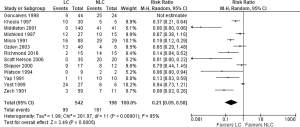
There was significant heterogeneity (Tau2 =1.04, Chi2 =69.70, df =10, P<0.00001: I2 =86%) among included studies. In the random effects model analysis (RR, 0.66; 95% CI, 0.33, 1.32; z =1.18; P=0.24. Figure 4), there was no statistically significant difference between two groups.
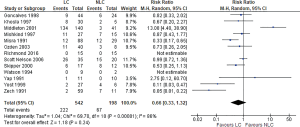
No resolution of symptoms
There was significant heterogeneity (Tau2 =1.91, Chi2 =40.16, df =11, P<0.0001: I2 =73%) among included trials. In the random effects model analysis (RR, 0.15; 95% CI, 0.05, 0.39; z =3.82; P=0.0001: Figure 5), the risk of failure to resolve symptoms was lower in LC group.
Discussion
LC may be considered as an acceptable surgical intervention in patients with biliary dyskinesia presenting with chronic right upper quadrant pain. Currently there is insufficient evidence to recommend the routine use of LC in every patient with biliary dyskinesia. Paucity of high power randomised, controlled trials is the major reason for this lack of the evidence which should be addressed sooner and until then current study may be used to provide basis for offering the LC in the selected group of patients.
Our understanding of biliary dyskinesia has improved recently, partly because of the efforts to define it more clearly (3). It is now believed to be a gallbladder motility disorder hence the understanding that decreased emptying on cholescintigraphy supports the diagnosis (25,36). This dysmotility disorder of the gallbladder has been associated with multiple intrinsic and extrinsic factors influencing gallbladder function such as gallbladder neuronal problems, diabetes mellitus, liver cirrhosis and chronic gallbladder inflammation etc. (34). Various studies have reported that the gallbladder histology in these patients showed chronic inflammation (25). Despite this knowledge and an expanding horizon on its aetio-pathogenesis, patients with biliary dyskinesia represent an exhausted group of patients who have been withstanding with this biliary colic type pain despite multiple health-care encounters and an array of blood and radiological investigations (36).
Current study has shown that LC may result in significant symptomatic improvement in patients with biliary dyskinesia. This is supported by several other reported studies (9,37-40). With much improved safety profile than of open cholecystectomy, LC these days can be a considerable option for the management of symptomatic biliary dyskinesia. The data is lacking as to which of these patients will benefit the most from LC (37). Some published literature suggests that reproduction of characteristic biliary colic type pain and low ejection fraction on cholescintigraphy is a good indicator of symptomatic improvement after LC (39,41).
There are several limitations of this study. Combined analysis of randomised and non-randomised studies is not an ideal way of achieving high quality evidence but due to the paucity of decent number of randomised trials, authors decided to include all types of studies to achieve relatively better evidence. Significant heterogeneity among included studies may be due to the diverse inclusion and exclusion criteria. Other potential sources of heterogeneity and biased outcome include the varying protocols of performing cholescintigraphy, lack of an agreed definition of biliary dyskinesia and lack of standard technique of LC in reported/included studies. The future implication of this study is to consider running a major multicentre randomised, controlled trial with agreed definition and diagnostic pathway of biliary dyskinesia, and a standard post-operative tool to accurately measure the symptomatic relief in patients after LC (42). Until then the current study may be used as baseline evidence to offer LC in a group of symptomatic patients with biliary dyskinesia.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep 2011;13:188-92. [Crossref] [PubMed]
- Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am 2010;39:369-79. x. [Crossref] [PubMed]
- Krishnamurthy S, Krishnamurthy GT. Biliary dyskinesia: role of the sphincter of Oddi, gallbladder and cholecystokinin. J Nucl Med 1997;38:1824-30. [PubMed]
- Richmond BK. Optimum utilization of cholecystokinin cholescintigraphy (CCK-HIDA) in clinical practice: an evidence based review. W V Med J 2012;108:8-11. [PubMed]
- DiBaise JK, Richmond BK, Ziessman HA, et al. Cholecystokinin-cholescintigraphy in adults: consensus recommendations of an interdisciplinary panel. Clin Nucl Med 2012;37:63-70. [Crossref] [PubMed]
- Goussous N, Kowdley GC, Sardana N, et al. Gallbladder dysfunction: how much longer will it be controversial? Digestion 2014;90:147-54. [Crossref] [PubMed]
- Krishnamurthy GT, Krishnamurthy S, Brown PH. Constancy and variability of gallbladder ejection fraction: impact on diagnosis and therapy. J Nucl Med 2004;45:1872-7. [PubMed]
- Ponsky TA, DeSagun R, Brody F. Surgical therapy for biliary dyskinesia: a meta-analysis and review of the literature. J Laparoendosc Adv Surg Tech A 2005;15:439-42. [Crossref] [PubMed]
- Mahid SS, Jafri NS, Brangers BC, et al. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatobiliary iminodiacetic acid scan results without gallstones. Arch Surg 2009;144:180-7. [Crossref] [PubMed]
- Sabbaghian MS, Rich BS, Rothberger GD, et al. Evaluation of surgical outcomes and gallbladder characteristics in patients with biliary dyskinesia. J Gastrointest Surg 2008;12:1324-30. [Crossref] [PubMed]
- Higgins JPT Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011:2011. Available online: http://handbook-5-1.cochrane.org/
- Review Manager (RevMan) (Computer program). Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, 2014.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139-45. [Crossref] [PubMed]
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341-50. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Egger M. Systematic Reviews in Health Care: Meta-Analysis in Context, Second Edition. 2008 17 Mar 2008.
- Deeks JJ, Altman DG, Bradburn MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta‐Analysis. 2017:285-12.
- Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training 2017. Available online: http://training.cochrane.org/handbook
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Chalmers TC, Smith H Jr, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49. [Crossref] [PubMed]
- Cochrane IMS. Available online: (accessed on 20/04/2017).http://ims.cochrane.org/revman/otherresources/ gradepro/download
- Goncalves RM, Harris JA, Rivera DE. Biliary dyskinesia: natural history and surgical results. Am Surg. 1998;64:493-7:discussion 497-8.
- Khosla R, Singh A, Miedema BW, et al. Cholecystectomy alleviates acalculous biliary pain in patients with a reduced gallbladder ejection fraction. South Med J 1997;90:1087-90. [Crossref] [PubMed]
- Middleton GW, Williams JH. Diagnostic accuracy of 99Tcm-HIDA with cholecystokinin and gallbladder ejection fraction in acalculous gallbladder disease. Nucl Med Commun 2001;22:657-61. [Crossref] [PubMed]
- Mishkind MT, Pruitt RF, Bambini DA, et al. Effectiveness of cholecystokinin-stimulated cholescintigraphy in the diagnosis and treatment of acalculous gallbladder disease. Am Surg. 1997;63:769-74. [PubMed]
- Misra DC Jr, Blossom GB, Fink-Bennett D, et al. Results of surgical therapy for biliary dyskinesia. Arch Surg 1991;126:957-60. [Crossref] [PubMed]
- Scott Nelson R, Kolts R, Park R, et al. A comparison of cholecystectomy and observation in children with biliary dyskinesia. J Pediatr Surg 2006;41:1894-8. [Crossref] [PubMed]
- Ozden N, Di Baise JK. Gallbladder ejection fraction and symptom outcome in patients with acalculous biliary-like pain. Dig Dis Sci 2003;48:890-7. [Crossref] [PubMed]
- Richmond BK, Grodman C, Walker J, et al. Pilot Randomized Controlled Trial of Laparoscopic Cholecystectomy vs Active Nonoperative Therapy for the Treatment of Biliary Dyskinesia. J Am Coll Surg 2016;222:1156-63. [Crossref] [PubMed]
- Skipper K, Sligh S, Dunn E, et al. Laparoscopic cholecystectomy for an abnormal hepato-iminodiacetic acid scan: a worthwhile procedure. Am Surg 2000;66:30-2. [PubMed]
- Watson A, Better N, Kalff V, et al. Cholecystokinin (CCK)-HIDA scintigraphy in patients with suspected gall-bladder dysfunction. Australas Radiol 1994;38:30-3. [Crossref] [PubMed]
- Yap L, Wycherley AG, Morphett AD, et al. Acalculous biliary pain: cholecystectomy alleviates symptoms in patients with abnormal cholescintigraphy. Gastroenterology 1991;101:786-93. [Crossref] [PubMed]
- Yost F, Margenthaler J, Presti M, et al. Cholecystectomy is an effective treatment for biliary dyskinesia. Am J Surg 1999;178:462-5. [Crossref] [PubMed]
- Zech ER, Simmons LB, Kendrick RR, et al. Cholecystokinin enhanced hepatobiliary scanning with ejection fraction calculation as an indicator of disease of the gallbladder. Surg Gynecol Obstet 1991;172:21-4. [PubMed]
- Lennard TW, Farndon JR, Taylor RM. Acalculous biliary pain: diagnosis and selection for cholecystectomy using the cholecystokinin test for pain reproduction. Br J Surg 1984;71:368-70. [Crossref] [PubMed]
- Geiger TM, Awad ZT, Burgard M, et al. Prognostic indicators of quality of life after cholecystectomy for biliary dyskinesia. Am Surg 2008;74:400-4. [PubMed]
- Wybourn CA, Kitsis RM, Baker TA, et al. Laparoscopic cholecystectomy for biliary dyskinesia: Which patients have long term benefit? Surgery 2013;154:761-7; discussion 767-8. [Crossref] [PubMed]
- Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech 2009;19:222-6. [Crossref] [PubMed]
- Hassler KR, Jones MW. Gallbladder, Cholecystectomy, Laparoscopic. StatPearls. Treasure Island (FL): StatPearls PublishingStatPearls Publishing LLC: 2017.
- Morris-Stiff G, Falk G, Kraynak L, et al. The cholecystokin provocation HIDA test: recreation of symptoms is superior to ejection fraction in predicting medium-term outcomes. J Gastrointest Surg 2011;15:345-9. [Crossref] [PubMed]
- Cotton P, Morgan K, Bingener J, et al. Cholecystectomy for Gallbladder Dyskinesia. J Am Coll Surg 2016;223:204-5. [Crossref] [PubMed]
Cite this article as: Rehman S, Singh KK, Sajid MS. Role of laparoscopic cholecystectomy in the management of chronic right upper quadrant pain due to biliary dyskinesia: a systematic review and meta-analysis. Transl Gastroenterol Hepatol 2019;4:71.