
Pathology of pancreatic cancer
Introduction
Neoplasms of the pancreas comprise a broad spectrum and are generally classified according to their histological differentiation as epithelial or non-epithelial and according to their biological behaviour in benign, pre-malignant or malignant neoplasms. Epithelial neoplasms can be either exocrine or endocrine, while the group of exocrine neoplasms is further classified in ductal and acinar neoplasms. An overview of pancreatic neoplasms is given in Table 1.

Full table
Pancreatic ductal adenocarcinoma (PDAC) is by far the most common type of pancreatic malignancy, accounting for about 90% of all pancreas neoplasms (1). Hence, the terms “pancreatic cancer” and “pancreatic ductal adenocarcinoma” are often used synonymously.
Macroscopy and grossing of PDAC
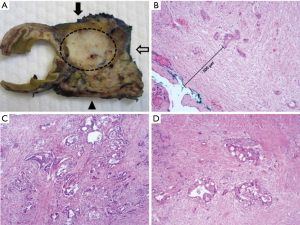
Most often, PDAC is located in the proximal pancreas, while an involvement of the pancreatic body or tail is rarer. In PDAC of the pancreatic head, obstruction of the common bile duct can lead to painless jaundice. However, PDAC is rarely diagnosed early. Usually, PDAC is between 2–4 cm at diagnosis (pT2), or even larger if located in the distal pancreas, and has already infiltrated surrounding structures (peripancreatic adipose tissue, duodenum, stomach, portal vein, etc.). It presents as solid and firm white-yellowish poorly-defined mass (Figure 1A). Regional lymph node metastases are also commonly present at diagnosis (2). The grossing of PDAC specimens is of great importance for the workup of a PDAC case, with the three main aspects being the extent of the primary tumor, which is relevant for the T category of the TNM staging, the presence and number of lymph node metastases and the relationship of the tumor to the resection margins. A highly standardized slicing and sampling technique with axial sectioning of the specimen perpendicular to the longitudinal axis of the descending duodenum is recommended for pancreaticoduodenectomy specimens. This enables correlation of macroscopic findings with CT or MRI imaging, as well as the evaluation of the circumferential resection margin (CRM) macroscopically and, subsequently, also on the microscopic slide (3,4). The CRM consists of the anterior, posterior and medial pancreatic surface. These three surfaces should be inked with different colors prior to sectioning, according to a pre-defined color code, so that the relationship of the tumor to each surface can be recapitulated on the microscopic specimens. Although the definition and nomenclature of the CRM is a subject of controversial debate, it is often affected by microscopically incomplete (R1) resections and should therefore always be evaluated (3,5) (Figure 1B). For distal pancreatectomy specimens, inking of the posterior and anterior surface and slicing perpendicular to the longitudinal axis of the pancreas is recommended, in order to enable evaluation of the CRM. Due to the dispersed growth of PDAC especially in the tumor periphery, the “1-mm rule” has been proposed to determine the R-status at the CRM: a true R0 situation (“R0 wide”) is only reported if no tumor cells are present within 1 mm of the respective resection margin. In case of tumor cells within 1 mm of the margin, but not within the margin itself, the resection is defined as (most appropriately) “R1-1 mm” or in alternative “R0 narrow” or “R0, CRM +”. If tumor cells are present directly within the margin, the resection is classified as “R1”. It has been shown in a large meta-analysis that patients with “R0 wide” status show a significantly better survival than patients with “R1 - 1 mm” and patients with “R1” status (6). Some studies even suggest a stricter cut-off for “R0 wide”, such as 1.5 or 2 mm (7,8). The standardized grossing approach also enables retrieval of a higher number of lymph nodes compared to non-standardized protocols, a relevant aspect considering the prognostic role of the so-called lymph node ratio (LNR) (ratio of metastatic to the total number of retrieved lymph nodes) (9).
Histomorphology of PDAC
Microscopically, PDAC consists of atypical tubular glands resembling medium-sized or smaller pancreatic ducts. However, growth patterns are strikingly heterogeneous among and within tumors. PDAC can include non-tubular components, such as a clear-cell, cribriform or gyriform component, which may have an impact on patient survival (10). The irregular tumor glands of PDAC are often—mostly in the case of well- and moderately differentiated PDAC—embedded in a prominent desmoplastic stroma, which consists of stromal cells, inflammatory cells and extracellular matrix proteins and contributes to the aggressive biological behavior of this neoplasm (Figure 1C) (11). Histopathological grading of PDAC is an important prognostic factor (12) and is performed according to defined WHO criteria, including the presence of tubular structures vs. solid growth, the presence of mucin, nuclear polymorphism and number of mitoses (13) (Table 2). The desmoplastic stromal reaction is less developed to absent in poorly differentiated PDAC.
Immunoprofile of PDAC
Although the diagnosis of PDAC can usually be made based on conventional histology, immunohistochemistry can be useful to distinguish PDAC from other primary tumors in case of metastases. PDAC usually express cytokeratins such as CK7, CK19, CK18 and sometimes CK20. CEA, CA19-9 and CA125, MUC1, MUC4 and MUC5AC are usually positive in PDAC as well. Staining for CEA and MUC1 can also be used to distinguish PDAC tumor glands from reactive ductular glands, as tumor glands usually show an apical and cytoplasmic expression, while reactive glands show no or only apical expression of the two markers.
Pathology of neoadjuvant treated PDAC
Although the importance of neoadjuvant treatment of PDAC is increasing, especially in primarily non-resectable and borderline-resectable PDAC, little to no standardization has been established both regarding therapy regimens and pathological evaluation of specimens. A multitude of tumor regression grading (TRG) systems have been proposed in the past and are currently in use in the assessment of therapeutic success. Most of the TRG systems are based on the semi-quantitative evaluation of destruction of viable cancer cells on one hand and the extent of therapy-induced fibrosis on the other hand. While these criteria are sufficient for other gastrointestinal cancers, major problems occur when applying these criteria in PDAC. Even modern imaging techniques are not reliable in determining the original tumor size of PDAC prior to therapy (14). However, without knowledge of the original tumor size, the percentage of destroyed cancer cells cannot be determined reliably. After resection, both gross and microscopic evaluation of the tumor extent is challenging in PDAC, even in untreated specimens, due to its dispersed growth, which is even more prominent in pre-operatively treated PDAC, as regression and therefore tumor-induced fibrosis may be patchy. Moreover, even therapy-naïve PDAC is characterized by a prominent stromal reaction, making the extent of therapy-induced fibrosis an unsatisfactory criterion for TRG. While efforts have been made to find markers that can help distinguish tumor-associated desmoplasia and therapy-induced fibrosis, no such markers have been found so far (15). Other morphological changes in neoadjuvant PDAC, which are sometimes used in TRG, include necrosis, inflammation, mucin pools and regressive changes the tumor cells, such as marked eosinophilia and vacuolization of the cytoplasm and high-grade nuclear atypia (Figure 1D). However, all these changes are not only hard to quantify, but may all be present in therapy-naïve PDAC as well.
The difficulties discussed above illustrate the urgent need to improve PDAC TRG. Various new aspects have recently been suggested to be incorporated in PDAC TRG. These include, for example, focusing on the residual cancer, e.g., with the implementation of a residual cancer burden score (taking into account the size and cellularity of the residual primary and the number and size of residual lymph node metastases), similar to what has been done in breast cancer, or the use of Ki67 immunohistochemistry to determine the proliferative activity of the residual cancer (16). In the future, meticulous and highly standardized assessment of pre-treated PDAC specimens is needed in order to validate existing and new aspects of PDAC TRG.
Variants of PDAC
A number of morphological variants of PDAC exist. While most share a similar molecular pathogenesis and therefore similar biological behaviour and prognosis with “classical” PDAC, some variants are characterized by a different molecular background and prognosis. Variants with a similar molecular pathogenesis include adenosquamous carcinoma, anaplastic (undifferentiated) carcinoma, undifferentiated carcinoma with osteoclastic giant cells, micropapillary carcinoma, signet-ring cell carcinoma and the large-duct type carcinoma of the pancreas. On the other hand, colloid carcinoma, medullary carcinoma and hepatoid adenocarcinoma of the pancreas are variants with a distinct molecular pathogenesis.
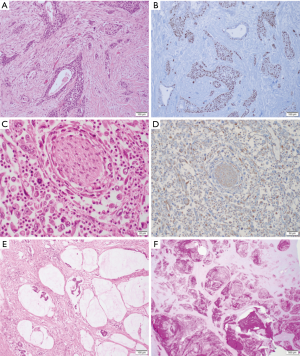
Adenosquamous carcinomas of the pancreas are defined as carcinomas with a squamous component making up at least 30% of the tumor mass, while the glandular component can be minimal (Figure 2A,B) (13). Although they share a similar molecular carcinogenesis, their prognosis is even worse than the prognosis of classical PDAC (17). Similarly, anaplastic (undifferentiated) carcinomas of the pancreas also have a poorer prognosis than classical PDAC (18). These tumors are characterized by solid or dispersed growth and the presence of large, strikingly polymorphous tumor cells, including multinuclear tumor giant cells (Figure 2C). In immunohistochemistry, the anaplastic cells often co-express pan-cytokeratin (Pan-CK) and vimentin and display a loss of e-cadherin (Figure 2D). Anaplastic pancreatic carcinomas with a rhabdoid differentiation have been shown to harbour SMARCB1 mutations, while being KRAS wildtype (19). Anaplastic pancreatic carcinomas are not to be confused with pancreatic undifferentiated carcinomas with osteoclastic giant cells. These tumors include histiocytic giant cells, which can be distinguished from tumor giant cells by positivity for CD68, and seem to bear a markedly better prognosis with a 5-year survival rate of 60% (20). Micropapillary carcinomas of the pancreas resemble those of the breast and consist of densely packed micropapillary cell clusters within clefts, with a typical “inside-out” pattern of MUC1 staining (positivity of the stroma-facing cell surface) and cytoplasmic positivity for e-cadherin and galectin-3 (21). Primary signet-ring cell carcinomas of the pancreas are exceedingly rare, and only few case reports have been published (22). For the diagnosis of signet-ring cell carcinoma, many authors require for 50% or more of the tumor mass to consist of signet-ring cells, characterized by large cytoplasmic mucin vacuoles, pushing the nucleus to the periphery of the cell. The large-duct type variant of PDAC forms large, sometimes dilated ducts, can mimic non-invasive cystic tumors of the pancreas and share a similar patient survival with classical PDAC (23). Colloid (mucinous) carcinomas of the pancreas are frequently associated with high-grade intestinal-type intraductal papillary neoplasms of the pancreas and are characterized by the presence of extracellular mucin aggregates (Figure 2E,F). Intestinal-type IPMN and colloid carcinomas often harbour distinct GNAS mutations and often additional KRAS mutations (24,25). With a 5-year survival rate of 50%, these tumors exhibit a markedly better prognosis than classical PDAC (26). Medullary carcinomas of the pancreas have a distinct syncytial growth pattern, show pushing invasion, often include areas of necrosis and may be associated with microsatellite instability, similar to colorectal counterparts (27,28). Lastly, the hepatoid carcinoma of the pancreas is exceedingly rare and is a mimic of hepatocellular carcinoma concerning its morphology and immunoprofile (29). Recently, Fign mutations have been identified in hepatoid carcinomas based on data generated through transposon-induced carcinogenesis in mice.
Molecular subtyping of PDAC
While the role of the four cancer-related genes KRAS, TP53, SMAD4 and CDKN2A in PDAC carcinogenesis has been well known for many years, the development of sophisticated high-throughput techniques has enabled a much more detailed molecular characterization of PDAC in recent years. In 2011, transcriptome analyses of PDAC tissue samples and human and murine PDAC cell lines performed by Collisson and colleagues have led to the proposal of three molecular subtypes of PDAC: (I) the classical, (II) the quasi-mesenchymal and (III) the exocrine-like subtype of PDAC (30). While the classical subtype is characterized by the expression of epithelial and adhesion-related genes, the quasi-mesenchymal subtype primarily expresses mesenchyme-related genes, while the exocrine-like subtype is defined by the expression of genes linked to digestive enzymes (30). Interestingly, these subtypes seem to be relevant for survival, with the best prognosis being attributed to the classical subtype and the worst to the quasi-mesenchymal subtype (30). In addition, PDAC cell lines of the classical subtype seem to be resistant to gemcitabine therapy, but sensitive to erlotinib, while PDAC cell lines of the quasi-mesenchymal subtype seem gemcitabine-sensitive, but erlotinib-resistant (30); however, studies regarding current therapy regimens, such as gemcitabine plus nab-paclitaxel or FOLFIRINOX, are yet missing. Five years after that, Bailey and colleagues were able to determine four molecular subtypes of PDAC based on whole exome sequencing and copy number variation (CNV) analysis, which partially overlapped with Collisson’s subtypes (31). Further molecular subtypes proposed in 2015 by Waddell and colleagues focused on genomic stability vs. instability (32). These subtypes may have implications for therapy, e.g., the marked genomic instability in the unstable subtype may suggest sensitivity to DNA-damaging therapeutics (32). In addition to the molecular subtyping of PDAC epithelial cells, Moffitt and colleagues successfully performed molecular subtyping of PDAC stroma, resulting in the proposal of a “normal” and an “activated” PDAC stroma subtype, with the “activated” subtype being linked to a worse prognosis (33).
While translating these findings into clinical applications in the context of patient stratification and precision medicine seems like an urgent next step to take, limitations should be considered. Although similarities between subtypes described by different authors exist, subtypes do not overlap perfectly, which may in part be a result of the material that was used for the analysis. Due to the distinct biology and histomorphology of PDAC, contamination of tumor tissue samples with stromal cells has to be taken into account. Moreover, validation studies have recently found evidence that Collisson’s exocrine-like (Bailey’s ADEX subtype, respectively) may have resulted from contamination of tumor tissue samples with normal acinar cells (34). In addition, the correlation between molecular and histological subtypes is mostly lacking in the above-mentioned studies, e.g., Bailey’s squamous molecular subtype does not correspond to a squamous differentiation on a histomorphological level. Integration of histomorphological and molecular data has suggested that there is indeed a prognostic relationship between them (10). Therefore, the next step should not only be the validation and optimization of molecular subtypes, but also the integration of histomorphological subtypes.
Precursors of PDAC
Precursor lesions of PDAC can be divided into microscopic and macroscopic precursors.
Microscopic precursors include pancreatic intraepithelial neoplasia (PanIN) and possibly atypical flat lesions (AFL). PanIN are small (<0.5 cm in diameter) mucinous-papillary intraepithelial neoplasms with a ductal phenotype and can be classified as low-grade PanIN or high-grade PanIN according to the grade of cellular and nuclear atypia (35). PanIN are commonly found in pancreas resection specimens, but increasingly in patients with PDAC (16% in normal pancreata vs. 82% in pancreata with PDAC) (36). PanIN have been extensively studied in mouse models, which have proven that the formation of PanIN can be induced by the activation of the KRAS oncogene alone (37). While KRAS mutations are very frequently found in low-grade PanIN and high-grade PanIN, mutations of CKN2A TP53 and SMAD4 are usually only found in high-grade PanIN, and at a much lower frequency than in invasive PDAC (38,39).
On the other hand, AFL are small tubular lesions consisting of flat to cuboidal epithelia with cytologic atypia, surrounded by reactive stroma, and have been described in mouse models and patients with a familial predisposition for pancreatic cancer (40).
Despite the ductal phenotype of these two microscopic PDAC precursors, acinar cells have been shown to be cells of origin for PanIN and AFL (41,42), giving rise to the concept of acinar-ductal metaplasia (ADM) and a “metaplasia-dysplasia sequence” in pancreatic carcinogenesis.
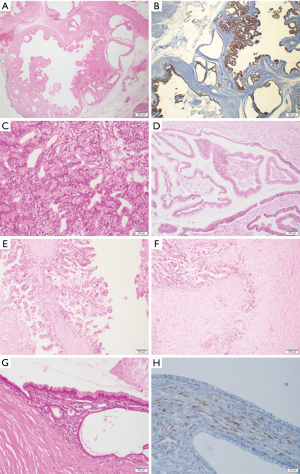
Macroscopic precursor lesions of PDAC include intraductal papillary mucinous neoplasms (IPMN) as well as mucinous cystic neoplasms (MCN) and intraductal tubulopapillary neoplasms (ITPN). IPMN are macroscopic (>1.0 cm in diameter) mucinous papillary intraepithelial neoplasms. They can be classified as main-duct, branch-duct or combined-type IPMN according to their site of origin and as gastric-type, intestinal-type and pancreatobiliary-type IPMN according to their histomorphology and immunoprofile (43-45) (Figure 3A,B,C,D,E,F). The fourth histological subtype of IPMN, oncocytic-type IPMN, is now recognized as a separate entity (intraductal oncocytic papillary neoplasm, IOPN) due to its unique biological behavior (46,47). Although IOPN most often display high-grade dysplasia and/or invasive carcinoma and tend to recur, their prognosis seems to be excellent after surgery (48). Main duct-type IPMN are usually intestinal-type IPMN or, more rarely, pancreatobiliary-type or gastric-type IPMN or IOPN, while branch-duct type IPMN are most commonly of gastric-type (26,49). An overview of the histomorphology and immunophenotypes of IPMN and IOPN is given in Table 3. Like PanIN, IPMN should be classified as high-grade or low-grade according to the grade of cytological atypia (35). In addition to KRAS mutations, GNAS mutations in codon 201 are typical for IPMN. While GNAS mutations may occasionally also be found in PanIN, they are much more frequent in IPMN and can be found in up to 2/3 IPMN cases (50). KRAS and GNAS mutations often occur simultaneously in IPMN, and GNAS mutations are found significantly more frequently in the intestinal subtype, whereas the KRAS mutations are significantly more common in the gastric subtype (51). Mutations of RNF43, which codes for a protein with intrinsic E3 ubiquitin ligase activity, also seem to be found in IPMN, although their clinicopathological significance remains to be elucidated (51,52). Similar to the situation in PanIN, mutations in CDKN2A, TP53 and SMAD4 are predominantly found in high-grade IPMN or IPMN associated with invasive carcinoma, respectively.

Full table
MCN are large cysts, which can be uni- or multilocular, often have a thick, sometimes calcified cyst wall and mostly occur in the distal pancreas of middle-aged women. Low-grade MCN are characterized by a single layer of mucinous epithelia with an underlying characteristic “ovarian-like” stroma, which stains positive for oestrogen and progesterone receptor in immunohistochemistry (Figure 3G,H) (53). In high-grade MCN, a more complex architecture with papillary projections and solid areas can be observed. On a molecular level, KRAS mutations are frequently found in low-grade and high-grade MCN (54), while GNAS mutations are usually absent in MCN (55). Like IPMN, MCN may harbour mutations in RNF43 (52). CDKN2A, TP53 and SMAD4 mutations are usually only found in high-grade MCN or MCN with associated invasive carcinoma.
Lastly, another rarer macroscopic PDAC precursor is ITPN. These lesions are connected to the pancreatic duct system like IPMN, but rarely produce mucin and therefore usually do not present as cystic lesions (56). Their architecture is mostly tubular, although papillary components are often also present. In most cases, ITPN are high-grade lesions. Unlike IPMN, ITPN do not harbor KRAS or GNAS mutations, while PIK3CA mutations seem more frequent in ITPN than in other intraductal neoplasms (57), underlining the notion that ITPN and IPMN are indeed different entities.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cascinu S, Falconi M, Valentini V, et al. Pancreatic cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v55-58. [Crossref] [PubMed]
- Peixoto RD, Speers C, McGahan CE, et al. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med 2015;4:1171-7. [Crossref] [PubMed]
- Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008;15:1651-60. [Crossref] [PubMed]
- Verbeke CS, Leitch D, Menon KV, et al. Anthoney, Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232-7. [Crossref] [PubMed]
- Menon KV, Gomez D, Smith AM, et al. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB 2009;11:18-24. [Crossref] [PubMed]
- Kim KS, Kwon J, Kim K, et al. Impact of Resection Margin Distance on Survival of Pancreatic Cancer: a Systematic Review and Meta-Analysis. Cancer Res Treat 2017;49:824-33. [Crossref] [PubMed]
- Gebauer F, Tachezy M, Vashist YK, et al. Resection margin clearance in pancreatic cancer after implementation of the Leeds Pathology Protocol (LEEPP): clinically relevant or just academic? World J Surg 2015;39:493-9. [Crossref] [PubMed]
- Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol 2009;27:2855-62. [Crossref] [PubMed]
- Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-74. [Crossref] [PubMed]
- Schlitter AM, Segler A, Steiger K, et al. Molecular, morphological and survival analysis of 177 resected pancreatic ductal adenocarcinomas (PDACs): Identification of prognostic subtypes. Sci Rep 2017;7:41064. [Crossref] [PubMed]
- Esposito I, Penzel R, Chaib-Harrireche M, et al. Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol 2006;208:673-85. [Crossref] [PubMed]
- Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011;254:311-9. [Crossref] [PubMed]
- Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 4th ed. WHO/IARC Classification of Tumours. Lyon: IARC Press, 2010:211-2.
- Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749-56. [Crossref] [PubMed]
- Haeberle L, Kapp AC, Esposito I, et al. Morphologic Characterization of Pancreatic Ductal Adenocarcinoma after Neoadjuvant Therapy Pancreatology 2017;17:S20. (Abstract).
- Verbeke C, Haberle L, Lenggenhager D, et al. Pathology assessment of pancreatic cancer following neoadjuvant treatment: Time to move on. Pancreatology 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Komatsu H, Egawa S, Motoi F, et al. Clinicopathological features and surgical outcomes of adenosquamous carcinoma of the pancreas: a retrospective analysis of patients with resectable stage tumors. Surg Today 2015;45:297-304. [Crossref] [PubMed]
- Clark CJ, Graham RP, Arun JS, et al. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. J Am Coll Surg 2012;215:627-34. [Crossref] [PubMed]
- Agaimy A, Haller F, Frohnauer J, et al. Pancreatic undifferentiated rhabdoid carcinoma: KRAS alterations and SMARCB1 expression status define two subtypes. Mod Pathol 2015;28:248-60. [Crossref] [PubMed]
- Muraki T, Reid MD, Basturk O, et al. Undifferentiated Carcinoma With Osteoclastic Giant Cells of the Pancreas: Clinicopathologic Analysis of 38 Cases Highlights a More Protracted Clinical Course Than Currently Appreciated. Am J Surg Pathol 2016;40:1203-16. [Crossref] [PubMed]
- Khayyata S, Basturk O, Adsay NV. Invasive micropapillary carcinomas of the ampullo-pancreatobiliary region and their association with tumor-infiltrating neutrophils. Mod Pathol 2005;18:1504-11. [Crossref] [PubMed]
- Yepuri N, Naous R, Richards C, et al. Poorly differentiated signet ring cell carcinoma of pancreas masquerading as chronic pancreatitis. J Surg Case Rep 2018;2018:rjy218. [Crossref] [PubMed]
- Bagci P, Andea AA, Basturk O, et al. Large duct type invasive adenocarcinoma of the pancreas with microcystic and papillary patterns: a potential microscopic mimic of non-invasive ductal neoplasia. Mod Pathol 2012;25:439-48. [Crossref] [PubMed]
- Tan MC, Basturk O, Brannon AR, et al. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J Am Coll Surg 2015;220:845-854.e1. [Crossref] [PubMed]
- Molin MD, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol 2013;20:3802-8. [Crossref] [PubMed]
- Furukawa T, Hatori T, Fujita I, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut 2011;60:509-16. [Crossref] [PubMed]
- Wilentz RE, Goggins M, Redston M, et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: A newly described and characterized entity. Am J Pathol 2000;156:1641-51. [Crossref] [PubMed]
- Goggins M, Offerhaus GJ, Hilgers W, et al. Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology. Poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+. Am J Pathol 1998;152:1501-7. [PubMed]
- Marchegiani G, Gareer H, Parisi A, et al. Pancreatic hepatoid carcinoma: a review of the literature. Dig Surg 2013;30:425-33. [Crossref] [PubMed]
- Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011;17:500-3. [Crossref] [PubMed]
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]
- Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. [Crossref] [PubMed]
- Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168-78. [Crossref] [PubMed]
- Puleo F, Nicolle R, Blum Y, et al. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology 2018;155:1999-2013.e3. [Crossref] [PubMed]
- Basturk O, Hong SM, Wood LD, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol 2015;39:1730-41. [Crossref] [PubMed]
- Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol 2003;16:996-1006. [Crossref] [PubMed]
- Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437-50. [Crossref] [PubMed]
- Feldmann G, Beaty R, Hruban RH, et al. Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg 2007;14:224-32. [Crossref] [PubMed]
- Hosoda W, Chianchiano P, Griffin JF, et al. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol 2017;242:16-23. [Crossref] [PubMed]
- Aichler M, Seiler C, Tost M, et al. Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: a comparative study in transgenic mice and human tissues. J Pathol 2012;226:723-34. [Crossref] [PubMed]
- Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007;11:291-302. [Crossref] [PubMed]
- von Figura G, Fahrenkrog-Petersen L, Hidalgo-Sastre A, et al. Atypical flat lesions derive from pancreatic acinar cells. Pancreatology 2017;17:350-3. [Crossref] [PubMed]
- Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;6:17-32. [Crossref] [PubMed]
- Salvia R, Crippa S, Partelli S, et al. Differences between main-duct and branch-duct intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg 2010;2:342-6. [Crossref] [PubMed]
- Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 2005;447:794-9. [Crossref] [PubMed]
- Basturk O, Chung SM, Hruban RH, et al. Distinct pathways of pathogenesis of intraductal oncocytic papillary neoplasms and intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch 2016;469:523-32. [Crossref] [PubMed]
- Basturk O, Tan M, Bhanot U, et al. The oncocytic subtype is genetically distinct from other pancreatic intraductal papillary mucinous neoplasm subtypes. Mod Pathol 2016;29:1058-69. [Crossref] [PubMed]
- Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Oncocytic-type intraductal papillary mucinous neoplasms: a unique malignant pancreatic tumor with good long-term prognosis. J Am Coll Surg 2015;220:839-44. [Crossref] [PubMed]
- Ban S, Naitoh Y, Mino-Kenudson M, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol 2006;30:1561-9. [Crossref] [PubMed]
- Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66. [Crossref] [PubMed]
- Lee JH, Kim Y, Choi JW, et al. KRAS, GNAS, and RNF43 mutations in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Springerplus 2016;5:1172. [Crossref] [PubMed]
- Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011;108:21188-93. [Crossref] [PubMed]
- Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 1999;23:410-22. [Crossref] [PubMed]
- Shibata H, Ohike N, Norose T, et al. Mucinous Cystic Neoplasms Lined by Abundant Mucinous Epithelium Frequently Involve KRAS Mutations and Malignant Progression. Anticancer Res 2017;37:7063-8. [PubMed]
- Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018;67:2131-2141. [Crossref] [PubMed]
- Yamaguchi H, Shimizu M, Ban S, et al. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2009;33:1164-72. [Crossref] [PubMed]
- Yamaguchi H, Kuboki Y, Hatori T, et al. The discrete nature and distinguishing molecular features of pancreatic intraductal tubulopapillary neoplasms and intraductal papillary mucinous neoplasms of the gastric type, pyloric gland variant. J Pathol 2013;231:335-41. [Crossref] [PubMed]
Cite this article as: Haeberle L, Esposito I. Pathology of pancreatic cancer. Transl Gastroenterol Hepatol 2019;4:50.



