Venous resection during pancreatectomy for pancreatic cancer: a systematic review
Introduction
Pancreatic cancer is one of the most aggressive and lethal malignancies with a dismal prognosis and survival. The overall 5-year survival is still as low as 8% (1). Even after radical resection, the 5-year survival is only up to 25% due to the high rate of recurrence, but however can also raise up to 40% in high-volume pancreatic cancer surgery centres (2,3). Fortunately, owing to improved chemotherapy regimens in combination with radical surgery, the 5-year survival rates in locally advanced cases have become as good as primarily resectable cases (4).
Therefore, surgical resection remains the only potentially curative option. Unfortunately, by the time of diagnosis, only a small portion (15–20%) of the newly diagnosed patients are suitable for surgical resection (5) due to local or distant metastases. The superior mesenteric/portal vein infiltration is often infiltrated, as the malignant tumor frequently invades into the retroperitoneal space and due to the intimate anatomic location of the uncinate process into the superior mesenteric or portal vein (6). In the past venous infiltration was considered as a contraindication for surgery due to the limitation of tumor dissection at the vessels. Today, with the development of better operation techniques, pancreatectomy combined with venous resection (VR) can be achieved with acceptable morbidity and mortality in experienced centers (7). Therefore, venous infiltration is no longer considered as a contraindication for pancreatic cancer resection. According to the NCCN Clinical Practice Guidelines in Oncology 2017 (8), the presence of solid tumor encasement of the superior mesenteric vein or portal vein >180° is considered as a criterion for defining the borderline resectable disease. However, the curative effects of VR in pancreatic cancer remain controversial.
In this study, we aimed to investigate the most up-to-date survival data and the perioperative outcome and survival associated with pancreatectomy with superior mesenteric/portal vein resection, and compared it to patients without VR.
Methods
Search strategy
Quality items of the systematic review were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic literature search was performed in PubMed, Embase and the Cochrane Library by applying various combinations of the terms related to pancreatic cancer and VR. The search items were “pancreatic cancer”, “pancreatic ductal adenocarcinoma”, superior mesenteric vein” and “portal vein”. Articles published until May 2018 were included. No language restriction was applied in the search strategy.
Inclusion and exclusion criteria
For inclusion into the present systematic review, a study had to meet the following criteria:
- To compare the results of pancreatectomy with versus without VR in patients with pancreatic cancer;
- To report at least one outcome of interest.
Animal studies, case reports, review articles without original data, letters, comments, abstracts, duplicate reports and studies that contained non-cancer patients were excluded from the systematic review. Studies that contained arterial resection were also excluded, since arterial resection is frequently associated with impaired survival.
Data extraction
The following data were extracted from the full texts and supplemental materials: first author, year of publication, period of patient inclusion, title, study design, mean age, perioperative outcomes, inclusion criteria and exclusion criteria, patient characteristics, median survival and 1-, 2-, 3-, and 5-year survival. The perioperative outcomes included: overall postoperative complications (due to inconsistency in the definition of morbidity or even missing definitions, postoperative complications in general was provided instead), mortality, re-operation rate, sample size, hospital stay, duration of operation, blood loss and histopathology finding.
Statistical analysis
The Review Manager (RevMan, the Cochrane Collaboration) software version 5.3 was used for the data pooling. Dichotomous variables with the estimation of risk ratio (RR) or odds risk (OR) together with a 95% CI and continuous variables with weighted mean difference (WMD) and a 95% CI were analyzed. Pooled effect was calculated using either the fixed effects model or the random-effects model based on data features. Statistical heterogeneity between trials was evaluated by I2 and P value. If I2 was less than 50%, the fixed effects model was used as the absence of heterogeneity. Otherwise, the random effects model was applied if I2 exceeded 50%. P value <0.05 was considered significant. Publication bias was assessed visually with a funnel plot.
Results
Included studies
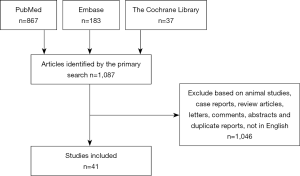
A total of 1,087 articles were retrieved by the primary search. After screening the titles, abstracts, and full text, 1,046 studies were excluded and 41 studies (9-30) were eligible for inclusion in the review (Figure 1) (31-49). There were no randomized clinical trials on the subject. The 41 studies involved 7,567 patients in total; among these, 1,921 underwent pancreatectomy with and 5,646 without VR.
Characteristics of the studied patients
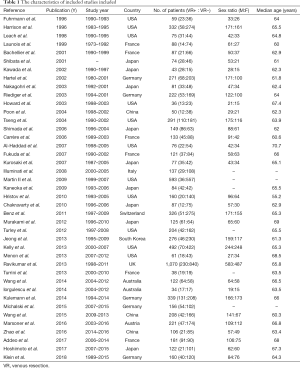
The characteristics of all the included studies and the baseline demographic data of the enrolled patients were summarized in Table 1. The studies were published between the years 1996 and 2018, with the sample size varying from a minimum of 34 to a maximum of 1,070. The VR rate ranged from 6.1% to 65.1%. The mean age of the patients was 64.5 years.
Full table
Perioperative outcomes
Data on duration of surgery were available in 11 (13,17,21,23,28,30,37,43,44,46,47) of 41 studies which demonstrated a prolonged operating time in patients with VR. The mean operating time was 491 minutes (ranging from 342 to 667) for patients undergoing pancreatectomy with VR, compared to 399 minutes (ranging from 306 to 568) for patients without VR (P<0.00001). Meanwhile, data on blood loss during operation was available only in 8 studies (17,21,23,28,34,37,44,46). The average blood loss in the VR+ group was 929 mL (ranging from 343 to 1,686) and in the VR− group 581 mL (ranging from 353 to 866), indicating VR with increased blood loss (P=0.0001).
Data on postoperative mortality (refers to death within 30 days after surgery) was reported in 28 studies, involving 5,773 patients. Compared to patients without VR (3.17%, range 0–13.51%), mortality in VR+ patients was increased (3.84%, range 0–13.73%; P=0.03). Whereas, data on 90-day morbidity was available on 3 studies (42,44,47), involving 728 patients. There is also no significant difference between two groups (P=0.27).
A total of 19 retrospective cohort studies (3,499 patients) (9,14,15,19,21,22,25-32,34,36,37,41,47) revealed that the overall postoperative complications showed no significant difference between the two groups (P=0.07). The overall postoperative complications in the VR+ group was 37% (range 20.7–55.6%), compared to the VR− group (34%, range 19.4–63.6%). To allow a more detailed insight, data of wound infection, abdominal abscesses, postoperative bleeding and delayed gastric emptying were extracted. Pooled analysis revealed that the risk of wound infection (P=0.42) and intra-abdominal abscess (P=0.31) were similar between the two groups. By contrast, the risks of postoperative bleeding (P<0.0001) and delayed gastric emptying (P=0.03) were markedly higher in the VR+ group. Interestingly, patients who underwent pancreatectomy with VR+ revealed less pancreatic fistula (7.9%, range 0–16.3%), than patients without VR− (10.7%, range 2.47–33.3%; P=0.0010).
Seventeen studies (9,10,13,17,20,21,23,27,28,33,37,40,42,43,46,47,49), including 2,469 patients, reported about re-operation rates. Two hundred eighty three patients underwent re-operations, 104 in the VR+ group and 179 in the VR− group, respectively. Pooled analysis of data found that the overall incidence of re-operation in the VR+ group was 12.3% (ranging from 0% to 48%), which was higher when compared to VR− group (11.0% ranging from 0% to 25.33%; P=0.008).
Histopathology
Nine studies (13,17,21,30,34,37,41,46,47), containing 1,445 patients in total, reported the tumor size as a prognostic variable. The average tumor dimension in the VR+ group was 35.7 mm (range, 28.2–47.9 mm), and the dimension in VR− group was 30.8 mm (range, 26.7–41 mm). Patients receiving VR+ were more likely to have bigger tumors than those without VR− (P<0.00001).
Twenty-six studies, including 5,065 patients, reported data on R0 (negative margin) rate. The definition of R0 was determined by the authors of each study. Using a fixed-effects model, the incidence of R0 rate in VR+ group was markedly lower comparatively to VR− groups (60.5%, range 35.7–94.4% vs. 68.7%, 47.3–92.9%; P<0.00001).
Survival analysis
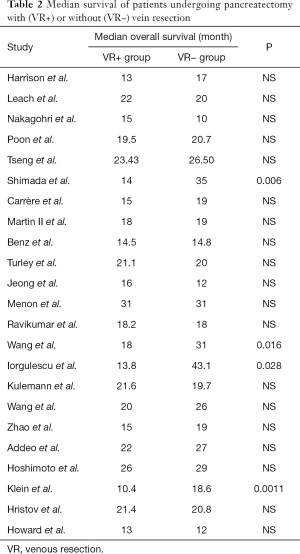
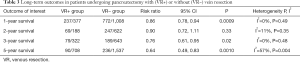
To evaluate the effect of VR, the median survival and the 1-, 2-, 3-, and 5-year overall survival were extracted and compared between the two groups. Data on median overall survival were available for 23 studies (Table 2). Four studies identified VR as a negative prognostic factor (P=0.006, P =0.016, P=0.028, and P=0.0011, respectively). Pooled analysis of data from 12 studies (11,19-21,33,35,39-41,44,48,49) showed that VR+ patients have significantly shorter median survival (P=0.0001). Furthermore, patients who received VR had worse 1- (13,24,25,30,31,39,44,45,47,48), 3- (18,24-26,30,37,39,47,48), and 5-year (10,14,15,18,21-24,36,39,45,47,48) survival (Table 3). Analysis of long-term survival revealed that 1-year survival rate was decreased in VR+ group (62.9%) compared to VR- group (76.6%) (P=0.0009). Similarly, 3-year survival rates and 5-year survival rates were lower in VR+ group (24.5% vs. 29.4% for 3-year survival, P=0.02 and 12.7% vs. 15.4% for 5-year survival, P=0.001).
Full table
Full table
Type of venous reconstruction
The venous reconstruction techniques included end-to-end anastomosis, patch venoplasty, venorrhaphy, and graft interposition. Data on the type of venous reconstruction were available for 18 studies (10,11,13,15-18,26-30,35,36,38,43,44,47) with 932 patients. Generally, the selection of different vessel reconstruction techniques depended on the length of the vessel infiltration. The most common reconstruction method was an end-to-end anastomosis (531 patients, 57.0%), which was normally performed by a continuous running 5-0 polypropylene suture. If the involvement of the vein was longer than 5 cm, an interposition vascular graft was considered in order to perform a tension-free anastomosis. Launois et al. (12) reported that a distance of up to 8 cm could be covered by end-to-end anastomosis after mobilizing of the mesenteric base. No data were available for the mortality, morbidity, and survival for the different types of venous reconstruction.
Discussion
This systematic review investigated the perioperative outcomes and long-term outcomes in patients suffered from pancreatectomy with or without VR. The study demonstrated the overall postoperative complications were similar between the two groups. Moreover, patients in the VR+ group showed a relatively higher mortality and worse 1-, 3-, 5-year survival.
Pancreatectomy combined with VR provides a possibility of a radical treatment option for patients with pancreatic cancer. Although VR can be performed feasibly and safely (50), the outcomes of VR remain discrepant. Pancreatectomy combined with VR was first reported by Moore et al. (51) in 1951. However, subsequent studies demonstrated that there was no survival benefit from VR. Moreover, VR was also associated with increased morbidity and mortality. Therefore, venous infiltration was considered as a contraindication to surgery for pancreatic cancer. With deepened understanding and development of operative techniques, pancreatectomy combined with VR can achieve similar morbidity and mortality compared to standard pancreatectomy.
In this study, we demonstrated that the overall postoperative complications were similar between the two groups, which underline that VR can be performed safely. Although the mortality is higher in the VR group, it is notable that these patients suffered from larger tumors, reduced R0 rates, longer operation time, as well as increased perioperative blood loss. Subgroup analysis for postoperative complications revealed that the risks of postoperative bleeding and delayed gastric emptying were markedly higher in the VR group, which is accordant with the fact that patients undergoing VR received relatively more complex surgery and had longer operation time. In line with our results, Carrère et al. (21) reported that the risk of pancreatic fistula was significantly decreased in the VR+ group, since patients in VR+ group revealed a more fibrotic pancreatic remnant. Furthermore, tumor topography and volume may also contribute to this phenomenon. Our study demonstrated that patients in VR+ group tended to have larger tumors, which consequently may lead to more frequent obstruction and a dilated main-pancreatic duct, which enables a safe anastomosis.
Regarding histopathology, there was no significant difference between VR- and VR+ group in the rate of lymph node metastasis. However, compared with VR- group, patients in VR+ group tended to suffer from larger tumors and lower R0 resection rate, which may imply that they tended to have more aggressive and malignant tumors. Riediger et al. (22) investigated patients from both groups with margins negative or margins positive specimens, and reported markedly increased survival in margins negative group. Consistent with our previous study (2), we found that R1 resection in pancreatic head resection frequently associated with impaired survival both in the meta-analysis and in our cohort. Moreover, patients with R1 resection were suffering from advanced tumor disease, including larger tumor size, high rate of lymph node metastasis. Similar benefits of radical resection were also revealed by Neuhaus et al. (52) for hilar cholangiocarcinoma. Hence, radical excision with large tumor-free margins remains to be essential for solid tumors.
Consistent with some other meta-analyses, our findings demonstrate that VR during pancreatectomy is associated with worse survival (53-55). The shorter survival of patients who suffered from VR seems to be attributable to larger tumor size and the higher rate of positive margins. Yu et al. (55) reported in 2014 that the VR+ group and the VR− group had similar 1-year survival and 3-year survival. Yet at 5-year, VR+ group showed a worse survival (P=0.03). The higher number of patients and possibly more advanced disease of the involved patients in our study may explain the different results that are obtained here. More importantly, the data points out that patients with negative margin have significantly better 2- and 5-year survival (55). VR provides the opportunity of achieving R0 resection for the patients with venous infiltration, following with an obvious survival advantage (5-year survival: 25%) compared to the palliative setting only (5-year survival: 7%). Therefore, pancreatectomy combined with VR seems to be justified in the patients with vein infiltration since VR can offer the chance of radical margin-free surgery.
There are several limitations in this systematic review. First, 5 studies (56-60) were excluded because data on VR could not be separated from arterial resection in these studies. Furthermore, the heterogeneity of the included studies is another limitation of this review. The studies included in this systematic review were published from 1996 to 2018. The techniques of surgery and perioperative care have since been further developed and changed including different protocols of adjuvant chemotherapy. As a consequence, the results of short-term and long-term outcomes might have been influenced by this long time window. Moreover, besides these developments, the existence of surgical experience and protocol disparities in different surgery centres can also contribute to statistical heterogeneity. Another limitation is that randomized controlled trial (RCT) is not available on this topic, since VR is necessary to achieve R0 resection in patients with vein infiltration and it is highly impossible to randomize patients into VR− and VR+ groups.
In conclusion, VR in pancreatic cancer is a safe and feasible procedure. Given the great benefit of R0 resection and the fact that patients have miserable outcomes and survival in the palliative setting only, extended resection including VR is required for the purpose of achieving radical resection, which is considered to be the best option to achieve long-term survival for patients with solid tumors. Future studies with larger case series from high-volume pancreatic cancer surgery centres are necessary to demonstrate the true impact of VR, especially when regarding novel neoadjuvant therapy protocols.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Demir IE, Jager C, Schlitter AM, et al. R0 Versus R1 Resection Matters after Pancreaticoduodenectomy, and Less after Distal or Total Pancreatectomy for Pancreatic Cancer. Ann Surg 2018;268:1058-68. [Crossref] [PubMed]
- Siegel R, Ma JM, Zou ZH, et al. Cancer Statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic Adenocarcinoma. N Engl J Med 2014;371:1039-49. [Crossref] [PubMed]
- Tseng JF, Tamm ER, Lee JE, et al. Venous resection in pancreatic cancer surgery. Best Pract Res Clin Gastroenterol 2006;20:349-64. [Crossref] [PubMed]
- Müller SA, Hartel M, Mehrabi A, et al. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg 2009;13:784-92. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028-61. [Crossref] [PubMed]
- Fuhrman GM, Leach SD, Staley CA, et al. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric portal vein confluence. Ann Surg 1996;223:154-62. [Crossref] [PubMed]
- Harrison LE, Klimstra DS, Brennan MF. Isolated portal vein involvement in pancreatic adenocarcinoma - A contraindication for resection? Ann Surg 1996;224:342-7. [Crossref] [PubMed]
- Leach SD, Lee JE, Charnsangavej C, et al. Survival following pancreaticoduodenectomy with resection of the superior mesenteric-portal vein confluence for adenocarcinoma of the pancreatic head. Br J Surg 1998;85:611-7. [Crossref] [PubMed]
- Launois B, Stasik C, Bardaxoglou E, et al. Who benefits from portal vein resection during pancreaticoduodenectomy for pancreatic cancer? World J Surg 1999;23:926-9. [Crossref] [PubMed]
- Bachellier P, Nakano H, Oussoultzoglou E, et al. Is pancreaticoduodenectomy with mesentericoportal venous resection safe and worthwhile? Am J Surg 2001;182:120-9. [Crossref] [PubMed]
- Shibata C, Kobari M, Tsuchiya T, et al. Pancreatectomy combined with superior mesenteric-portal vein resection for adenocarcinoma in pancreas. World J Surg 2001;25:1002-5. [Crossref] [PubMed]
- Hartel M, Niedergethmann M, Farag-Soliman M, et al. Benefit of venous resection for ductal adenocarcinoma of the pancreatic head. Eur J Surg 2002;168:707-12. [Crossref] [PubMed]
- Kawada M, Kondo S, Okushiba S, et al. Reevaluation of the indications for radical pancreatectomy to treat pancreatic carcinoma: is portal vein infiltration a contraindication? Surg Today 2002;32:598-601. [Crossref] [PubMed]
- Howard TJ, Villanustre N, Moore SA, et al. Efficacy of venous reconstruction in patients with adenocarcinoma of the pancreatic head. J Gastrointest Surg 2003;7:1089-95. [Crossref] [PubMed]
- Nakagohri T, Kinoshita T, Konishi M, et al. Survival benefits of portal vein resection for pancreatic cancer. Am J Surg 2003;186:149-53. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Pancreaticoduodenectomy with en bloc portal vein resection for pancreatic carcinoma with suspected portal vein involvement. World J Surg 2004;28:602-8. [Crossref] [PubMed]
- Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8:935-49; discussion 49-50. [Crossref] [PubMed]
- Carrère N, Sauvanet A, Goere D, et al. Pancreaticoduodenectomy with mesentericoportal vein resection for adenocarcinoma of the pancreatic head. World J Surg 2006;30:1526-35. [Crossref] [PubMed]
- Riediger H, Makowiec F, Fischer E, et al. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg 2006;10:1106-15. [Crossref] [PubMed]
- Shimada K, Sano T, Sakamoto Y, et al. Clinical implications of combined portal vein resection as a palliative procedure in patients undergoing pancreaticoduodenectomy for pancreatic head carcinoma. Ann Surg Oncol 2006;13:1569-78. [Crossref] [PubMed]
- Al-Haddad M, Martin JK, Nguyen J, et al. Vascular resection and reconstruction for pancreatic malignancy: A single center survival study. J Gastrointest Surg 2007;11:1168-74. [Crossref] [PubMed]
- Fukuda S, Oussoultzoglou E, Bachellier P, et al. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch Surg 2007;142:172-9. [Crossref] [PubMed]
- Illuminati G, Carboni F, Lorusso R, et al. Results of a pancreatectomy with a limited venous resection for pancreatic cancer. Surg Today 2008;38:517-23. [Crossref] [PubMed]
- Kurosaki I, Hatakeyama K, Minagawa M, et al. Portal vein resection in surgery for cancer of biliary tract and pancreas: Special reference to the relationship between the surgical outcome and site of primary tumor. J Gastrointest Surg 2008;12:907-18. [Crossref] [PubMed]
- Kaneoka Y, Yamaguchi A, Isogai M. Portal or superior mesenteric vein resection for pancreatic head adenocarcinoma: prognostic value of the length of venous resection. Surgery 2009;145:417-25. [Crossref] [PubMed]
- Martin RCG, Scoggins CR, Egnatashvili V, et al. Arterial and Venous Resection for Pancreatic Adenocarcinoma Operative and Long-term Outcomes. Arch Surg 2009;144:154-9. [Crossref] [PubMed]
- Chakravarty KD, Hsu JT, Liu KH, et al. Prognosis and feasibility of en-bloc vascular resection in stage II pancreatic adenocarcinoma. World J Gastroenterol 2010;16:997-1002. [Crossref] [PubMed]
- Hristov B, Reddy S, Lin SH, et al. Outcomes of Adjuvant Chemoradiation after Pancreaticoduodenectomy with Mesenterico-Portal Vein Resection for Adenocarcinoma of the Pancreas. Int J Radiat Oncol Biol Phys 2010;76:176-80. [Crossref] [PubMed]
- Banz VM, Croagh D, Coldham C, et al. Factors influencing outcome in patients undergoing portal vein resection for adenocarcinoma of the pancreas. Eur J Surg Oncol 2012;38:72-9. [Crossref] [PubMed]
- Turley RS, Peterson K, Barbas AS, et al. Vascular Surgery Collaboration During Pancreaticoduodenectomy With Vascular Reconstruction. Ann Vasc Surg 2012;26:685-92. [Crossref] [PubMed]
- Kelly KJ, Winslow E, Kooby D, et al. Vein Involvement During Pancreaticoduodenectomy: Is There a Need for Redefinition of "Borderline Resectable Disease"? J Gastrointest Surg 2013;17:1209-17. [Crossref] [PubMed]
- Menon VG, Puri VC, Annamalai AA, et al. Outcomes of Vascular Resection in Pancreaticoduodenectomy: Single-surgeon Experience. Am Surg 2013;79:1064-7. [PubMed]
- Murakami Y, Uemura K, Sudo T, et al. Benefit of portal or superior mesenteric vein resection with adjuvant chemotherapy for patients with pancreatic head carcinoma. J Surg Oncol 2013;107:414-21. [Crossref] [PubMed]
- Turrini O, Ewald J, Barbier L, et al. Should the Portal Vein Be Routinely Resected During Pancreaticoduodenectomy For Adenocarcinoma? Ann Surg 2013;257:726-30. [Crossref] [PubMed]
- Ravikumar R, Sabin C, Abu Hilal M, et al. Portal Vein Resection in Borderline Resectable Pancreatic Cancer: A United Kingdom Multicenter Study. J Am Coll Surg 2014;218:401-11. [Crossref] [PubMed]
- Wang F, Gill AJ, Neale M, et al. Adverse Tumor Biology Associated with Mesenterico-Portal Vein Resection Influences Survival in Patients with Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 2014;21:1937-47. [Crossref] [PubMed]
- Iorgulescu DG, Ling S, Nikfarjam M, et al. Results of pancreatic resection associated with portal vein resection in an Australian tertiary care centre. ANZ J Surg 2015;85:270-3. [Crossref] [PubMed]
- Jeong J, Choi DW, Choi SH, et al. Long-term outcome of portomesenteric vein invasion and prognostic factors in pancreas head adenocarcinoma. ANZ J Surg 2015;85:264-9. [Crossref] [PubMed]
- Kulemann B, Hoeppner J, Wittel U, et al. Perioperative and Long-Term Outcome after Standard Pancreaticoduodenectomy, Additional Portal Vein and Multivisceral Resection for Pancreatic Head Cancer. J Gastrointest Surg 2015;19:438-44. [Crossref] [PubMed]
- Michalski CW, Kong B, Jager C, et al. Outcomes of resections for pancreatic adenocarcinoma with suspected venous involvement: a single center experience. BMC Surg 2015;15:100. [Crossref] [PubMed]
- Wang WL, Ye S, Yan S, et al. Pancreaticoduodenectomy with portal vein/superior mesenteric vein resection for patients with pancreatic cancer with venous invasion. Hepatobiliary Pancreat Dis Int 2015;14:429-35. [Crossref] [PubMed]
- Marsoner K, Langeder R, Csengeri D, et al. Portal vein resection in advanced pancreatic adenocarcinoma: is it worth the risk? Wien Klin Wochenschr 2016;128:566-72. [Crossref] [PubMed]
- Zhao X, Li LX, Fan H, et al. Segmental portal/superior mesenteric vein resection and reconstruction with the iliac vein after pancreatoduodenectomy. J Int Med Res 2016;44:1339-48. [Crossref] [PubMed]
- Addeo P, Velten M, Averous G, et al. Prognostic value of venous invasion in resected T3 pancreatic adenocarcinoma: Depth of invasion matters. Surgery 2017;162:264-74. [Crossref] [PubMed]
- Hoshimoto S, Hishinuma S, Shirakawa H, et al. Reassessment of the clinical significance of portal-superior mesenteric vein invasion in borderline resectable pancreatic cancer. Eur J Surg Oncol 2017;43:1068-75. [Crossref] [PubMed]
- Klein F, Berresheim F, Felsenstein M, et al. Routine portal vein resection for pancreatic adenocarcinoma shows no benefit in overall survival. Eur J Surg Oncol 2018;44:1094-9. [Crossref] [PubMed]
- Fortner JG. Regional Resection of Cancer of Pancreas - New Surgical Approach. Surgery 1973;73:307-20. [PubMed]
- Moore GE, Sako Y, Thomas LB. Radical Pancreatoduodenectomy with Resection and Reanastomosis of the Superior Mesenteric Vein. Surgery 1951;30:550-3. [PubMed]
- Neuhaus P, Thelen A, Jonas S, et al. Oncological Superiority of Hilar En Bloc Resection for the Treatment of Hilar Cholangiocarcinoma. Ann Surg Oncol 2012;19:1602-8. [Crossref] [PubMed]
- Bell R, Ao BT, Ironside N, et al. Meta-analysis and cost effective analysis of portal-superior mesenteric vein resection during pancreatoduodenectomy: Impact on margin status and survival. Surg Oncol 2017;26:53-62. [Crossref] [PubMed]
- Giovinazzo F, Turri G, Katz MH, et al. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg 2016;103:179-91. [Crossref] [PubMed]
- Yu XZ, Li J, Fu DL, et al. Benefit from synchronous portal-superior mesenteric vein resection during pancreaticoduodenectomy for cancer: a meta-analysis. Eur J Surg Oncol 2014;40:371-8. [Crossref] [PubMed]
- Cheung TT, Poon RTP, Chok KSH, et al. Pancreaticoduodenectomy with vascular reconstruction for adenocarcinoma of the pancreas with borderline resectability. World J Gastroenterol 2014;20:17448-55. [Crossref] [PubMed]
- Delpero JR, Boher JM, Sauvanet A, et al. Pancreatic Adenocarcinoma with Venous Involvement: Is Up-Front Synchronous Portal-Superior Mesenteric Vein Resection Still Justified? A Survey of the Association Fran double dagger aise de Chirurgie. Ann Surg Oncol 2015;22:1874-83. [Crossref] [PubMed]
- Hwang JW, Kim SC, Song KB, et al. Significance of Radiologic Location and Extent of Portal Venous Involvement on Prognosis After Resection for Pancreatic Adenocarcinoma. Pancreas 2015;44:665-71. [Crossref] [PubMed]
- Fang JZ, Lu CD, Wu SD, et al. Portal vein/superior mesenteric vein resection in pancreatic cancer treatment in the elderly. Medicine (Baltimore) 2017;96:e7335. [Crossref] [PubMed]
- Kleive D, Sahakyan MA, Berstad AE, et al. Trends in indications, complications and outcomes for venous resection during pancreatoduodenectomy. Br J Surg 2017;104:1558-67. [Crossref] [PubMed]
Cite this article as: Wang X, Demir IE, Schorn S, Jäger C, Scheufele F, Friess H, Ceyhan GO. Venous resection during pancreatectomy for pancreatic cancer: a systematic review. Transl Gastroenterol Hepatol 2019;4:46.