
Peritransplant kinetics of Mac-2-binding protein glycosylation isomer levels in living donor liver transplantation: its implication of posttransplant small-for-size syndrome
Introduction
Wisteria floribunda agglutinin positive human Mac-2 binding protein glycosylation isomer (M2BPGi) has recently developed as a noninvasive serum marker of liver fibrosis (1). M2BPGi is known as a glycoprotein specific to humans. Therefore, the analyses of its biological behavior absolutely rely on measuring human specimens. Although liver transplant candidates with advanced cirrhosis have high serum levels of M2BPGi, the kinetics of M2BPGi after liver transplantation has remained to be elucidated so far. The aim of the current study was to elucidate the posttransplant kinetics of M2BPGi and the correlation between M2BPGi levels and posttransplant liver function.
Methods
This study was approved by the institutional review board of Kyushu University (No. 29-420). Fifteen recipients who underwent living donor liver transplantation (LDLT) between June 2015 and January 2016 and whose pretransplant, postoperative day (POD) 1, POD 3, and POD 7 sera were available for measuring M2BPGi were enrolled in this study.
The graft selection strategies and the perioperative management of donors and recipients were done as previously described (2,3). Five left grafts and 10 right grafts (without the middle haptic vein trunk) were used. Our standard operative procedures were as follows: on the donor side, hepatic parenchyma was transected using CUSATM (Valleylab Inc., Boulder, CO) under intermittent Pringle’s maneuver. Intraoperative cholangiography was performed in order to decide the cutting points of the hepatic ducts. After the completion of parenchymal transection and division of the hepatic duct, the portal veins, the hepatic arteries, and the hepatic veins on the graft sides were divided at each apposite level and then the hepatic grafts were procured. The grafts were perfused with UW solution (ViaspanTM, DuPont Pharmaceutical, Wilmington, DE) (4). The grafts were transplanted into the recipients without veno-venous bypass. After total hepatectomy, the hepatic veins on the grafts were sutured to the extended right hepatic veins or the extended confluence of the left and the middle hepatic veins using continuous 5-0 monofilament absorbable sutures. When there were any significant venous orifices of the middle hepatic vein tributaries on a right graft, these were reconstructed mainly using the internal jugular vein grafts (5). After the portal veins were reconstructed using continuous 6-0 monofilament absorbable sutures, the grafts were reperfused. The hepatic arteries were reconstructed using interrupted 8-0 monofilament non-absorbable sutures under a microscope (6). Biliary reconstructions were performed by duct-to-duct anastomosis using interrupted 6-0 monofilament absorbable sutures (7).
The levels of serum M2BPGi were measured by a lectin-antibody sandwich immunoassay using a fully automatic immunoanalyzer (HISCL-5000; Sysmex Co., Kobe, Japan) (8,9). Small-for-size syndrome (SFSS) was defined as the presence of cholestasis (total bilirubin >10 mg/dL) on POD 7 and intractable ascites (>1 L/day on POD14 or >500 mL/day on POD 28) without other specific causes (10).
The Wilcoxon rank-sum test was used for comparing variables. Proportions were compared using the Fisher exact test. Correlations between continuous variables were analyzed by the Spearman rank correlation test. A cutoff value was determined at the point where the sum of its sensitivity and its specificity was maximum in the receiver operating characteristic curve (11). Statistical significance was defined as having a P value of <0.05. All statistical analyses were performed using the NCSS software package (12).
Results
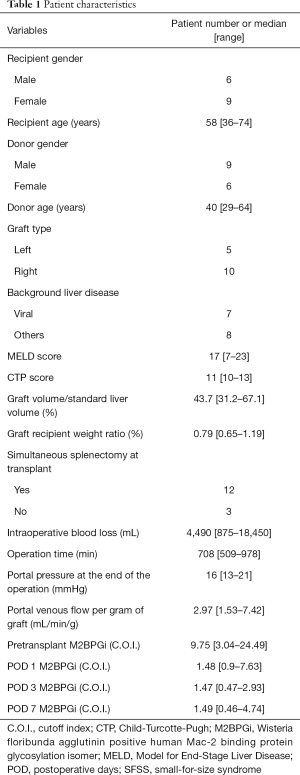
The characteristics of the patients are summarized in Table 1. The pretransplant serum levels of M2BPGi were high in all recipients, reflecting severe fibrosis of their livers (Figure 1).
Full table
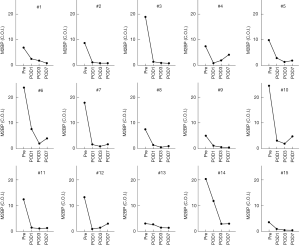
There was neither any significant correlation between pretransplant Model for End-stage Liver Disease scores and pretransplant M2BPGi levels (r=0.416, P=0.123), nor between pretransplant Child-Turcott-Pugh scores and pretransplant M2BPGi levels (r=−0.221, P=0.428) (Figure 2).
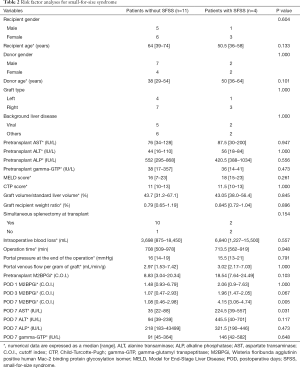
The levels decreased dramatically on POD 1 after LDLT (Figure 1). However, the levels increased again on POD 7 in some recipients (Patient #4, #6, #10, and #12 in Figure 1). We divided the 15 recipients into recipients without SFSS (n=11) and recipients with SFSS (n=4) and compared several risk factors for SFSS between the two groups (Table 2). We previously reported that recipient age ≤45 and donor age ≥48 were significant risk factors for SFSS (3). Although there were trends towards being significant in these two risk factors, they did not reach statistical significance (P=0.133 and P=0.101, respectively). The two significant risk factors among the variables examined were POD 7 aspartate transaminase (AST) and POD 7 M2BPGi. POD 7 AST and POD 7 M2BPGi in recipients with SFSS were significantly higher than those in recipients without SFSS (P=0.031 and 0.005, respectively).
Full table
All the 4 recipients with POD 7 M2BPGi more than 3.00 C.O.I developed SFSS. When the cutoff of POD 7 M2BPGi for predicting SFSS was determined to be 3.06 according to the receiver operating characteristic curve, all the sensitivity, the specificity, the accuracy, the positive predictive value, and the negative predictive value were 100%.
The basic strategy for SFSS was to treat symptoms until the graft regenerates. Two of the four patients with SFSS underwent plasma exchange. One patient died of resultant multiple organ failure 4 months after LDLT. The other three patients recovered without re-transplantation and were alive at the last follow-up (follow-up time ranging from 3 years 4 months to 3 years 9 months). All patients without SFSS were alive at the last follow-up (follow-up time ranging from 3 years 2 months to 3 years 10 months).
Discussion
To the best of our knowledge, the present study is the first one that investigated the posttransplant kinetics of M2BPGi in liver transplantation and showed a predictive value of M2BPGi for SFSS. Initially, M2BPGi was reported to be a novel glycoprotein for liver fibrosis. Thereafter, there have been several reports regarding other properties of M2BPGi than predicting liver fibrosis (13-16). Okuda et al. (13) suggested that M2BPGi was a useful predictor of posthepatectomy liver failure. Kono et al. (14) suggested that measuring serum M2BPGi was a potential biomarker in patients with idiopathic pulmonary fibrosis. Morio et al. (15) and Yamada et al. (16) recently reported that M2BPGi increases in patients with acute liver injury, suggesting this novel marker reflect not only liver fibrosis but also other factors, such as liver inflammation, liver damage like acute cellular rejection, and hepatocyte regeneration. The decrease of liver compliance induced by acute cellular or humoral rejection might be one of the causes of SFSS. In fact, one recipient (Patient #6 in Figure 1) suffered SFSS probably induced by acute humoral rejection. In the current study, all the 4 recipients with M2BPGi >3.0 cutoff index (C.O.I.) on POD 7 developed SFSS later. The cause of SFSS is multifactorial, rendering understanding pathophysiology of SFSS difficult. The probable representative causes are hyperperfusion of liver grafts, prolonged portal hypertension and resultant bacterial translocation, arterial hypoperfusion and so on. Which factor might contribute to the increased levels of M2BPGi on POD 7 in recipients who later developed SFSS? Does the production of M2BPGi increase or does the clearance of this glycoprotein decrease in such recipients? Recently, Bekki et al. (17) revealed in their in vitro studies that hepatic stellate cells were the source of M2BPGi. They also suggested that M2BPGi be an activation marker of hepatic stellate cells as a juxtacrine-acting messenger that makes hepatic stellate cells mutually work together with Kupffer cells during liver fibrosis. Therefore, once a cirrhotic liver is replaced with a new liver by liver transplantation, activated stellate cells and activated Kupffer cells in the cirrhotic liver are also replaced with inactivated stellate cells and inactivated Kupffer cells both of which reside in the new liver, which may cause the dramatic decrease of M2BPGi after liver transplantation. When a grafted liver is damaged by some reasons that lead to SFSS, hepatic stellate cells, the source of M2BPGi, might be activated by some of the contributory mechanisms of SFSS. Then, that mutual interrelationship between hepatic stellate cells and Kupffer cells may be strengthened again and, as a result, the levels of M2BPGi might re-rise.
Unfortunately, we could not reveal the half-life of M2BPGi. The half-life is affected by the status of the transplanted liver and the amount of blood loss/transfusion during liver transplantation and so on. The analyses of M2BPGi’s biological behavior absolutely rely on measuring human specimens because M2BPGi is specific to humans. There are many things to be elucidated with regard to the pathophysiology of M2BPGi in relation to liver transplantation.
There have been other reported early markers of graft dysfunction than M2BPGi. Rostved et al. (18) suggested that hyaluronic acid be a biomarker for allograft dysfunction. Selten et al. (19) demonstrated in their porcine model that the release of microRNA-122 during liver preservation was associated with early allograft dysfunction and graft survival after transplantation. Gorgen et al. (20) and Zulian et al. (21) revealed that serum factor V could be an early predictor of graft dysfunction after liver transplantation. The predictive power of early graft dysfunction by posttransplant M2BPGi levels may be weak compared to these previously reported markers because M2BPGi would rise after POD3. Rather, the re-rise of M2BPGi on POD 7 suggested the later development of SFSS.
There are shortcomings of the current study. First, the sample size is small, consisting of only 15 recipients. There may be some bias including a type II error. With the increase of sample size, variables such as donor age and pretransplant M2BPGi levels may become statistically significant. Although the results presented here were relatively distinct irrespective of the small sample size, studying the kinetics of M2BPGi in a large cohort may uncover further significant roles of such interesting glycoprotein in liver transplant settings. Second, measuring M2BPGi on POD7 is a post-transplant factor. Some investigators might insist that posttransplant factors should not be used for predicting post-hepatectomy dysfunction. However, M2BPGi must be a rapid-turnover glycoprotein as evidenced by the dramatic decrease after liver transplantation shown in Figure 1 and some posttransplant conditions such as rejection may cause SFSS. Therefore, M2BPGi can be an earlier marker for predicting SFSS than other potential posttransplant predictors.
In conclusion, M2BPGi has dramatic kinetics after liver transplantation and can be a predictive marker for small-for-size syndrome.
Acknowledgments
Funding: This work was supported by Japan Society for the Promotion of Science, KAKENHI (16K15606 and 16K10575).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Kyushu University (No. 29-420).
References
- Shirabe K, Bekki Y, Gantumur D, et al. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol 2018;53:819-26. [Crossref] [PubMed]
- Uchiyama H, Shirabe K, Nakagawara H, et al. Revisiting the safety of living liver donors by reassessing 441 donor hepatectomies: is a larger hepatectomy complication-prone? Am J Transplant 2014;14:367-74. [Crossref] [PubMed]
- Uchiyama H, Shirabe K, Kimura K, et al. Outcomes of adult-to-adult living donor liver transplantation in 321 recipients. Liver Transpl 2016;22:305-15. [Crossref] [PubMed]
- Ikegami T, Harimoto N, Shimokawa M, et al. The learning curves in living donor hemiliver graft procurement using small upper midline incision. Clin Transplant 2016;30:1532-7. [Crossref] [PubMed]
- Uchiyama H, Yoshizumi T, Ikegami T, et al. Use of internal jugular vein grafts in reconstructing multiple venous orifices of right hepatic grafts without the middle hepatic vein trunk. Liver Transpl 2017;23:110-6. [Crossref] [PubMed]
- Uchiyama H, Shirabe K, Yoshizumi T, et al. Use of living donor liver grafts with double or triple arteries. Transplantation 2014;97:1172-7. [Crossref] [PubMed]
- Ikegami T, Shimagaki T, Kawasaki J, et al. Eversion technique to prevent biliary stricture after living donor liver transplantation in the universal minimal hilar dissection era. Transplantation 2017;101:e20-5. [Crossref] [PubMed]
- Yamasaki K, Tateyama M, Abiru S, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014;60:1563-70. [Crossref] [PubMed]
- Kuno A, Ikehara Y, Tanaka Y, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013;3:1065. [Crossref] [PubMed]
- Soejima Y, Taketomi A, Yoshizumi T, et al. Feasibility of left lobe living donor liver transplantation between adults: an 8-year, single-center experience of 107 cases. Am J Transplant 2006;6:1004-11. [Crossref] [PubMed]
- Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristics curves. Acta Paediatr 2007;96:644-7. [Crossref] [PubMed]
- Hintze J: NCSS 11. 2016 Kaysville, UT: NCSS LLC. Available online: http://www.ncss.com. Accessed 1 January 2019.
- Okuda Y, Taura K, Yoshino K, et al. Usefulness of Mac-2 binding protein glycosylation isomer for prediction of posthepatectomy liver failure in patients with hepatocellular carcinoma. Ann Surg 2017;265:1201-8. [Crossref] [PubMed]
- Kono M, Nakamura Y, Oyama Y, et al. Increased levels of serum Wisteria floribunda agglutinin-positive Mac-2 binding protein in idiopathic pulmonary fibrosis. Respir Med 2016;115:46-52. [Crossref] [PubMed]
- Morio K, Imamura M, Daijo K, et al. Wisteria floribunda agglutinin positive Mac-2-binding protein level increases in patients with acute liver injury. J Gastroenterol 2017;52:1252-7. [Crossref] [PubMed]
- Yamada N, Katano T, Hirata Y, et al. Serum Mac-2 binding protein glycosylation isomer predicts the activation of hepatic stellate cells after liver transplantation. J Gastroenterol Hepatol 2019;34:418-24. [Crossref] [PubMed]
- Bekki Y, Yoshizumi T, Shimoda S, et al. Hepatic stellate cells secreting WFA+-M2BP: its role in biological interactions with Kupffer cells. J Gastroenterol Hepatol 2017;32:1387-93. [Crossref] [PubMed]
- Rostved AA, Ostrowski SR, Peters L, et al. Hyaluronic acid is a biomarker for allograft dysfunction and predicts 1-year graft loss after liver transplantation. Transplant Proc 2018;50:3635-43. [Crossref] [PubMed]
- Selten JW, Verhoeven CJ, Heedfeld V, et al. The release of microRNA-122 during liver preservation is associated with early allograft dysfunction and graft survival after transplantation. Liver Transpl 2017;23:946-56. [Crossref] [PubMed]
- Gorgen A, Prediger C, Prediger JE, et al. Serum Factor V is a continuous biomarker of graft dysfunction and a predictor of graft loss after liver transplantation. Transplantation 2019;103:944-51. [PubMed]
- Zulian MC, Chedid MF, Chedid AD, et al. Low serum factor V level: early predictor of allograft failure and death following liver transplantation. Langenbecks Arch Surg 2015;400:589-97. [Crossref] [PubMed]
Cite this article as: Uchiyama H, Shirabe K, Bekki Y, Toshima T, Harimoto N, Ikegami T, Yoshizumi T. Peritransplant kinetics of Mac-2-binding protein glycosylation isomer levels in living donor liver transplantation: its implication of posttransplant small-for-size syndrome. Transl Gastroenterol Hepatol 2019;4:41.


