
Treatment of pancreatic cancer—neoadjuvant treatment in borderline resectable/locally advanced pancreatic cancer
Introduction
Malignant tumors of the pancreas—mainly pancreatic ductal adenocarcinoma (PDAC)—confer a significant cancer burden to the population with an incidence of 12.1/100,000 and a mortality of 11.1/100,000 (1). In the Western world and in Germany, PDAC is currently ranked fourth place for causing cancer-related deaths (1,2). Furthermore, due to the increasing prevalence of PDAC and the stagnation in treatment strategies, PDAC is expected to reach rank two of cancer-related deaths by the year of 2030. If a curative treatment is pursued, a pancreaticoduodenectomy (PD, Whipple’s procedure) has to be performed. In this regard, a R0 resection, preferably with a tumor-free resection margin of ≥1 mm, is mandatory and is associated with a significantly improved survival rate (3,4). Furthermore, to improve the outcome, the incidence of perioperative complications has to be minimized and, if occurring, has to be treated sufficiently. This sets high demands for disciplines such as interventional radiology and intensive care as well. Through these means, long-term survival and cure of the disease can be achieved (5). Unfortunately, this is not achieved in most cases (6).
Borderline resectable PDAC
In resectable PDAC, a five-year survival rate of 40% can be reached after a R0 resection followed by adjuvant chemotherapy with Gemcitabine and Capecitabine (7). However, whereas only 10–20% of patients have primarily resectable PDAC on first presentation, 50–60% are diagnosed with metastasized PDAC (8). A certain group of PDAC features borderline resectable pancreatic cancers, which are mainly defined by their contact with major vessels as the celiac trunk, the superior mesenteric artery (9) or the portal vein and the superior mesenteric vein (SMV) (Table 1; Figure 1). Several classifications define borderline resectability. A cancer contact of the superior mesenteric artery (SMA) (9) of <180° is classified as borderline resectable according to the Americas Hepatopancreaticobiliary Association/Society for Surgery of the Alimentary Tract/Society of Surgical Oncology classification (AHPBA/SSO/SSAT Classification), the National Comprehensive Cancer Network classification (NCCN-Classification) and the classification of MD Anderson et al. (10,11). Concerning the celiac trunk, the MD Anderson classification allows for a contact of <180°, whereas the other classifications exclude tumors with any contact. For the common hepatic artery, all classifications allow contact of <180° or a short encasement of >180°. The AHPBA/SSO/SSAT Classification allows for an encasement of the portal vein or the SMV of >180° or obstruction of the vessel, whereas MD Anderson allows for an obstruction and the NCCN Classification allows for an involvement of <180° with narrowing for borderline resectable PDAC. Also, the exact localization of the infiltration site is important. For venous infiltration, a unilateral right-sided infiltration was shown to be associated with a better outcome and R0 resection rate than bilateral infiltration or left-sided infiltration (11). The current guidelines in Germany (“S3-Leitlinie”) for borderline resectable PDAC line up with the NCCN Classification (Table 1) (12). These classifications for borderline resectable PDAC are not equally accepted by all surgeons. The missing evaluation of longitudinal involvement (short, long distance) especially confers an important limitation to the classifications. In addition, if a subject has several definitions, this underlines the still existing controversy on this topic.

Full table
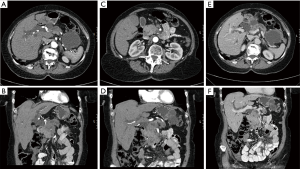
Evaluation of resectability is limited in preoperative imaging
For the evaluation of resectability, preoperative imaging by computed tomography (CT) or magnetic resonance imaging (MRI) is used, although it has limitations (13). In most cases, a CT scan is the standard preoperative imaging modality. Fong and coworkers were able to demonstrate that in 6% of patients with PDAC, there was no detectable cancer in preoperative CT imaging. Furthermore, 73% of the tumors with sizes of 1–2 cm were underestimated on initial imaging. Concerning involved lymph nodes, 30% of those classified as suspicious on imaging were negative in final pathological workup. Interestingly, these suspicious lymph nodes were associated with a preoperative biliary drainage (PBD; w/PBD: 63% vs. w/o PBD 37%; P=0.013) (14). Besides the evaluation of lymph node status, the evaluation of vessel involvement is also limited using CT scanning (13,14). The involvement of major vessels in preoperative CT scanning resulted in only 3% vessel resection, while 2% received a vessel resection without involvement of major vessels in preoperative CT scanning (14). A meta-analysis further underlined the high rates of false-positive evaluations of resectability in PDAC patients. For example, nineteen percent of the patients were predicted to be resectable by CT. They underwent exploration and were revealed as stage IV disease (distant metastasis) due to liver, lymph node and peritoneal metastasis. The positive predictive value of CT scanning for resectability was 81% (95% CI: 75–86%) (15). Endosonography (EUS) can also contribute information concerning vessel involvement. Here, a meta-analysis including 1,554 patients revealed advantages of EUS (AUC =0.9379) over CT (AUC =0.8589) scanning in predicting resectability of PDAC (16). Concerning EUS as an add-on diagnostic tool for predicting resectability for patients with resectable disease on CT, a Cochrane review showed limited benefits. Thirteen percent of the patients with unresectable EUS imaging had potentially resectable disease, whereas 20% with resectable EUS imaging had unresectable PDAC (17). Therefore, the authors concluded that additional EUS for patients with resectable disease on CT should not be performed routinely (17). Taken together, present available data underline, that approximately 20% of patients are staged incorrectly (under-/over-staged) by modern imaging, independent on which type of imaging is selected.
Neoadjuvant therapy for PDAC
An important part of today’s therapy of locally advanced or not primarily resectable PDAC is conferred by neoadjuvant therapy. In a retrospective study of 575 patients with locally advanced and not resectable PDAC, the effect of neoadjuvant therapy on resectability and outcomes was evaluated. The indication for neoadjuvant therapy was based on preoperative imaging in 335 patients, whereas the others presented with irresectable disease on exploration (42%). Here, irresectability was determined by arterial invasion (37%), venous invasion (11%), combined arterial and venous invasion (29%) and metastasis (24%). Neoadjuvant therapy was performed according to the FOLFIRINOX regime, radio chemotherapy with Gemcitabine or other treatment options. Neoadjuvant treatment resulted in resectability in 50.8% of the patients. Here, neoadjuvant therapy according to the FOLFIRINOX regime resulted in the highest rates of resectability (60.8%) compared with radio-chemotherapy with Gemcitabine or other treatment options (48.0%; P=0.0113). Resection resulted in improved survival: overall survival (15 vs. 8.5 months) and the three-year survival rate (23.0% vs. 2.4%) were both prolonged after resection compared with exploration alone (P<0.0001). Multivariate analysis revealed neoadjuvant treatment with FOLFIRINOX to be an independent predictor of improved prognosis (HR 0.68; P=0.0134), whereas irresectability due to metastasis in comparison with local irresectability was a negative predictor (HR 1.54; P=0.0016) (18). Due to the limitations of CT imaging in the evaluation of local tumor size and tumor vitality, the authors stated that patients without clear signs of progression of the disease during neoadjuvant treatment should undergo exploration (18,19). The central importance of neoadjuvant treatment in borderline resectable PDAC is further underlined by a recent meta-analysis demonstrating increased R0 resection rates after neoadjuvant treatment (R0: RR 1.13, P<0.00001; R1: RR 0.66, P<0.00001) (20). Concerning the achievement of a R0 resection, FOLFIRINOX (40.8%) outmatched Gemcitabine-based radio-chemotherapy (31.3%) and other regimes 27.3%; P=0.0408) (18). Achievement of an R0 resection also following neoadjuvant treatment depicts a fundamental prognostic factor. R0 resection was associated with a significantly improved five-year survival rate (37.7%) when compared with R1 with a tumor-free margin <1 mm (30.1%) or a direct R1 status (20.3%; P<0.0001) (3). Besides its beneficial effect on prognosis, neoadjuvant treatment of PDAC interestingly was associated with a reduced incidence of postoperative pancreatic fistula (OR 0.67; P<0.001) (21). Due to the beneficial effects of an R0 resection, the extent of the resection margin with resection of major veins (portal vein, SMV or splenic vein) may be necessary (Figure 2) (12). In contrast resection of arteries should be performed only in selected cases or study settings and not on a routine basis due to a higher postoperative morbidity and a five-times higher mortality (12). Most of the evidence on neoadjuvant treatment in borderline resectable PDAC is presently still based on retrospective studies. One recent randomized controlled trial compared neoadjuvant radio-chemotherapy based on Gemcitabine with upfront resection in patients with borderline resectable PDAC (22). After neoadjuvant radio-chemotherapy and resection (and vice versa in the upfront surgery group), patients received adjuvant treatment with Gemcitabine. The primary endpoint in this study was the two-year survival-rate. In the neoadjuvant-treated group, R0 resection was increased (52%) when compared with upfront surgery (26%; P=0.004) (22). Curative resection was possible in 71% of the patients. In this group, the tumor size was smaller (2.9±1.4 vs. 3.9±0.9 cm; P=0.014), and the number of positive lymph nodes was lower (n=0.5±0.9 vs. n=1.9±1.6; P=0.003) after neoadjuvant treatment when compared with upfront surgery. Furthermore, the R0 resection rate was significantly higher after neoadjuvant therapy (82.4% vs. 33.3%; P=0.010). The two-year survival was significantly increased after neoadjuvant treatment (41%) when compared with upfront surgery (26%; P=0.028). Recurrent disease was not different between neoadjuvant therapy (88%) or upfront resection (89%). Local recurrent disease occurred in 35% after neoadjuvant therapy and 28% after upfront resection, whereas systemic disease occurred in 71% after neoadjuvant therapy and 89% after upfront resection. Most recurrent disease was located in the lung (41% vs. 67%), whereas the liver was affected to a lower extent (5.9% vs. 5.6%) (22). The latest presented data on the benefits of neoadjuvant radio chemotherapy in borderline resectable and resectable PDAC were reported on ASCO 2018. In this randomized controlled trial patients with neoadjuvant radio-chemotherapy based on Gemcitabine were compared with upfront surgery, both followed by adjuvant therapy with Gemcitabine. The results revealed a significantly prolonged survival after neoadjuvant treatment (17.1 vs. 13.5 months; HR: 0.71; P=0.047) as well as an improved R0 resection rate (65% vs. 31%; P≤0.001) and a longer disease-free survival (11.2 vs. 7.9 months; HR: 0.67; P=0.010). Resection rates were similar between neoadjuvant treated patients and upfront surgery (62% vs. 72%; P=0.15). In resected patients, neoadjuvant treatment conferred a significant survival benefit when compared with upfront surgery (29.9 vs. 16.8 months; P<0.001) (23). Finally, a recent single-arm phase 2 study analyzed the effects of neoadjuvant FOLFIRINOX followed by individualized radio-chemotherapy in borderline resectable PDAC. Patients received eight cycles of FOLFIRINOX followed by either short-course radio-chemotherapy based on capecitabine in the case of the resolution of vessel invasion or long-course radio-chemotherapy based on capecitabine or fluorouracil in the case of persistent vessel invasion. 79% of the enrolled patients were able to complete all eight cycles of FOLFIRINOX, and 56% received short-course and 35% received long-course radio-chemotherapy. The R0 resection rate of all included patients (n=48) was 65%, whereas those who underwent resection (n=32) had a R0 resection rate of 97%. The median overall survival of all patients was 37.7 months, whereas the median survival of all resected patients was still not reached on the evaluation of the trial (24). These studies further emphasize the importance of neoadjuvant treatment in borderline resectable PDAC and call for further well-designed randomized studies also in resectable PDAC to improve evidence and thus patients’ outcomes. Here, promising studies as NEONAX (ClinicalTrials.gov identifier: NCT02047513) and NEOLAP (ClinicalTrials.gov Identifier: NCT02125136) are currently under way.
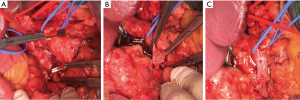
Conclusions
Based on current evidence, patients with borderline resectable and locally advanced PDAC should be evaluated for neoadjuvant treatment. There are recent randomized controlled trials supporting this neoadjuvant treatment concept. Here, neoadjuvant treatment with FOLFIRINOX as well as radio-chemotherapy based on Gemcitabine showed promising results. If there is no disease progression during neoadjuvant therapy, patients should undergo exploration with the aim of R0 resection, as re-staging CT or other imaging modalities have a low sensitivity for evaluating local resectability. Pancreaticoduodenectomy should be performed at a specialized center, and patients should be included in high-quality studies to achieve both the best possible patient outcomes and the generation of evidence from well-designed trials. If the neoadjuvant treatment concept is beneficial for patients with borderline resectable cancers, resectable PDAC has to be evaluated next in well-designed randomized controlled trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Koch-Institut, Z.f.K.i.R. 2018; Available online: https://www.krebsdaten.de/Krebs/SiteGlobals/Forms/Datenbankabfrage/datenbankabfrage_stufe2_form.html.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Strobel O, Hank T, Hinz U, et al. Pancreatic Cancer Surgery: The New R-status Counts. Ann Surg 2017;265:565-73. [Crossref] [PubMed]
- Demir IE, Jäger C, Schlitter AM, et al. R0 Versus R1 Resection Matters after Pancreaticoduodenectomy, and Less after Distal or Total Pancreatectomy for Pancreatic Cancer. Ann Surg 2018;268:1058-68. [Crossref] [PubMed]
- Howard TJ, Krug JE, Yu J, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointest Surg 2006;10:1338-45; discussion 1345-6. [Crossref] [PubMed]
- Carpelan-Holmström M, Nordling S, Pukkala E, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut 2005;54:385-7. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Russell R, Perkhofer L, Liebau S, et al. Loss of ATM accelerates pancreatic cancer formation and epithelial-mesenchymal transition. Nat Commun. 2015;6:7677. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028-61. [Crossref] [PubMed]
- Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013;20:2787-95. [Crossref] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, D.K., K. AWMF): S3-Leitlinie Exokrines Pankreaskarzinom, 2013 AWMF, and h.l.d.L.h. Registernummer: 032-010OL. Available online: https://www.awmf.org/uploads/tx_szleitlinien/032-010OLl_S3_Exokrines_Pankreaskarzinom_21112013-abgelaufen.pdf
- Egorov VI, Petrov RV, Solodinina EN, et al. Computed tomography-based diagnostics might be insufficient in the determination of pancreatic cancer unresectability. World J Gastrointest Surg 2013;5:83-96. [Crossref] [PubMed]
- Fong ZV, Tan WP, Lavu H, et al. Preoperative imaging for resectable periampullary cancer: clinicopathologic implications of reported radiographic findings. J Gastrointest Surg 2013;17:1098-106. [Crossref] [PubMed]
- Somers I, Bipat S. Contrast-enhanced CT in determining resectability in patients with pancreatic carcinoma: a meta-analysis of the positive predictive values of CT. Eur Radiol 2017;27:3408-35. [Crossref] [PubMed]
- Yang R, Lu M, Qian X, et al. Diagnostic accuracy of EUS and CT of vascular invasion in pancreatic cancer: a systematic review. J Cancer Res Clin Oncol 2014;140:2077-86. [Crossref] [PubMed]
- Tamburrino D, Riviere D, Yaghoobi M, et al. Diagnostic accuracy of different imaging modalities following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev 2016;9:CD011515. [PubMed]
- Hackert T, Sachsenmaier M, Hinz U, et al. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg 2016;264:457-63. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Schorn S, Demir IE, Reyes CM, et al. The impact of neoadjuvant therapy on the histopathological features of pancreatic ductal adenocarcinoma - A systematic review and meta-analysis. Cancer Treat Rev 2017;55:96-106. [Crossref] [PubMed]
- Cools KS, Sanoff HK, Kim HJ, et al. Impact of neoadjuvant therapy on postoperative outcomes after pancreaticoduodenectomy. J Surg Oncol 2018;118:455-62. [PubMed]
- Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018;268:215-22. [Crossref] [PubMed]
- Van Tienhoven G, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A randomized, controlled, multicenter phase III trial. J Clin Oncol 2018;36:LBA4002. [Crossref]
- Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:963-9. [Crossref] [PubMed]
Cite this article as: Scheufele F, Hartmann D, Friess H. Treatment of pancreatic cancer—neoadjuvant treatment in borderline resectable/locally advanced pancreatic cancer. Transl Gastroenterol Hepatol 2019;4:32.


