
Adjuvant treatment for pancreatic cancer
Introduction
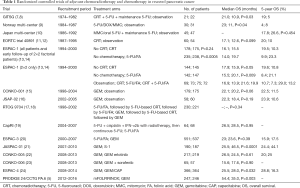
Pancreas cancer is meanwhile the third cause of cancer-associated mortality in Western countries with only an unsatisfying improvement in 5-year survival rates during the last decades to actually around 9% when considering all stages (1,2). Complete tumor removal offers the only chance for cure and upfront surgery therefore remains the treatment of choice in resectable tumors. In borderline resectable tumors with only venous involvement upfront surgery is also preferred, while for tumors with arterial involvement upfront resection is not routinely recommended (3). Only 15–20% of patients are candidates for immediate tumor resection, while the vast majority of patients are diagnosed with advanced metastatic disease and should therefore be considered for palliative treatment strategies (2,3). Surgery alone, however, does not enable long-term survival with median survival times of around 8–10 months and early tumor relapse in the majority of patients (2,4). Adjuvant chemotherapy has thus been developed during the last decades. As a result, postsurgical median survival has been more than doubled in selected patients receiving adjuvant chemotherapy with modified folinic acid, fluorouracil, irinotecan and oxaliplatin (mFOLFIRINOX), achieving median overall survival times of 54.4 months in the mFOLFIRINOX group (5). Until the 1990s, however, adjuvant therapy was not routinely recommended following resection. During the last three decades, enormous progress has been made in the conduct of high-quality, multicenter randomized controlled trials resulting in a radical change in onco-surgical management and treatment guidelines for resectable pancreatic cancer patients (6). This review article gives an overview of the development of adjuvant treatment for pancreatic cancer highlighting the most important clinical trials on this issue (Table 1).
Full table
Development of adjuvant treatment
Between 1974 and 1982, the first randomized controlled trial comparing adjuvant chemoradiotherapy and observation was conducted. Forty-three patients with microscopically tumor-negative resection margins were allocated to either radiotherapy with 40 Gy plus fluorouracil followed by maintenance chemotherapy with fluorouracil for 2 years, or to no adjuvant treatment (7). Due to poor accrual the trial was terminated prematurely. The median overall survival differed significantly among the two groups with longer survival times in the adjuvant chemoradiotherapy group (21.0 versus 10.9 months). To increase the sample size, additional 30 non randomized patients were included in the treatment group achieving a median overall survival of 18 months (8). However, the sample size was not sufficient to derive a convincing conclusion from this trial. The improved survival times in the chemoradiotherapy group may have been dominated by maintenance chemotherapy.
Bakkevold and colleagues then randomized 61 patients with resected pancreatic cancer (n=47) or carcinoma of the papilla of Vater (n=14) to adjuvant fluorouracil, doxorubicin plus mitomycin C once every 3 weeks for 6 cycles, or to no adjuvant treatment (9). Median overall survival was significantly prolonged in the adjuvant chemotherapy group (23 versus 11 months). Despite the multicenter study design, however, the a priori defined sample size of more than 80 patients could not be recruited, illustrating the difficulties in the conduct of randomized trials on adjuvant treatment at that time.
Between 1986 and 1992, a Japanese multicenter trial investigated the effect of adjuvant treatment with mitomycin C and fluorouracil versus surgery only in 508 patients with resected pancreatobiliary carcinomas (10). The intervention group received mitomycin C perioperatively and fluorouracil postoperatively until disease recurrence. Of 158 included patients with pancreatic cancer, 92 patients underwent curative resection. Five-year survival rates did not differ significantly among the two groups with even an increased survival rate in the observation group (17.8% versus 26.6%), making adjuvant chemotherapy still unconvincing.
Between 1987 and 1995, the EORTC gastrointestinal tract cancer cooperative group investigated the role of postoperative chemoradiotherapy (without maintenance chemotherapy) within a phase III clinical trial including 218 patients with pancreatic and ampullary cancer (11). When considering the pancreatic cancer subgroup only, there were 114 patients who were randomized to either the observation group (n=54) or to chemoradiotherapy (n=60) composed of radiotherapy with 40 Gy and fluorouracil. Median overall survival was not significantly different among the two groups. Non-superiority of the treatment group was confirmed when analyzing long-term follow-up data of patients included in this trial. The overall 10-year survival was 8% in the subgroup of pancreas head cancers with no significant differences among intervention and control group (12).
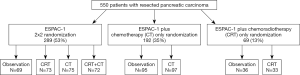
To further evaluate the effectiveness of adjuvant therapy in resected pancreatic cancer patients, the European Study Group for Pancreatic Cancer (ESPAC)-1 trial used a 2×2 factorial design to randomize 289 patients to either chemotherapy with fluorouracil/folinic acid for 6 cycles or no chemotherapy, and chemoradiotherapy with 20 Gy dose to the tumor given in ten daily fractions over a 2-week period plus fluorouracil or no chemoradiation (Figure 1). Additional 261 patients were allocated to either chemotherapy or chemoradiotherapy versus observation within the ESPAC-1 plus trial (13,14). After a median follow-up of 47 months of patients in the 2×2 factorial design, the median survival was 20.1 months among the 147 patients who received chemotherapy and 15.5 months among the 142 patients who did not receive chemotherapy. The results of the ESPAC-1 trial showed that adjuvant chemotherapy significantly increased survival compared to no chemotherapy, whereas survival was worse with chemoradiotherapy than with no chemoradiotherapy.
The CONKO-001 multicenter randomized trial evaluated 6 cycles of adjuvant chemotherapy with gemcitabine versus no adjuvant chemotherapy in 354 patients with resected pancreatic cancer and postoperative tumor markers (CEA and CA 19-9) of a maximum of 2.5 times the upper limit of the normal values (15). Disease-free (primary endpoint) survival was significantly longer in the treatment group compared to control group (13.4 versus 6.9 months), whereas median overall survival was comparable between the gemcitabine group and the control group (22.1 versus 20.2 months). Nevertheless, the results from the CONKO-001 trial favored adjuvant mono-chemotherapy with gemcitabine. The Japanese JSAP-02 trial compared 3 cycles of adjuvant monotherapy with gemcitabine versus no adjuvant chemotherapy in 118 Asian patients undergoing resection for pancreatic cancer (16). Median overall survival was comparable between the adjuvant chemotherapy and no adjuvant chemotherapy group (22.3 versus 18.4 months).
The RTOG 9704 phase III trial investigated the effect of the addition of gemcitabine to adjuvant chemoradiotherapy with fluorouracil on survival in patients with resected pancreatic cancer (17,18). Median overall survival of 388 patients with pancreatic head carcinoma was 20.5 months in the gemcitabine group versus 16.9 months in the fluorouracil group. When compared with the individual treatment groups in the ESPAC-1 trial one may suggest better survival times associated with chemotherapy than with chemoradiotherapy. The Journal of the National Cancer Institute then drew the conclusion that only few data were in favor of adjuvant chemoradiotherapy for pancreatic cancer (25).
Between 2004 and 2007, the CapRI trial investigated chemoradio-immunotherapy with fluorouracil, cisplatin, and interferon alfa-2b plus radiotherapy followed by 2 cycles of fluorouracil compared to 6 cycles of fluorouracil monotherapy in a total of 132 patients with resected pancreatic cancer (19). Median overall survival was comparable between patients receiving chemoradio-immunotherapy and those receiving fluorouracil monotherapy (26.5 versus 28.5 months). Considering substantial adverse effects in the chemoradio-immunotherapy, the authors concluded that this treatment cannot be recommended (19).
A head to head comparison between fluorouracil/folinic acid as used in ESPAC-1 and gemcitabine as used in CONKO-001 was undertaken within the ESPAC-3 trial (version 2). A total number of 1,088 patients was randomized to either adjuvant chemotherapy with gemcitabine or fluorouracil/folinic acid for 6 months after resection for pancreatic cancer (20). At a median follow-up time of 34.2 months, median overall survival times were similar between the two chemotherapy arms (23.0 months for fluorouracil/folinic acid versus 23.6 months for gemcitabine monotherapy) (20). However, grade 3/4 toxicities were almost halved in the gemcitabine group compared to the fluorouracil/folinic acid group (7.5% versus 14%). Consequently, from then on adjuvant gemcitabine was recommended as the treatment of choice in pancreatic cancer patients following upfront resection. It was shown that time to start chemotherapy did not influence overall survival rates for patients treated within ESPAC-3 (26). Based on the data from Vallee et al. the completion of all 6 cycles of adjuvant chemotherapy but not early start time is critical for postsurgical survival. Consequently, postponing chemotherapy for up to 12 weeks with the aim to allow adequate time for recovery and increase the chance of completing all 6 cycles may be more beneficial. These lesions learned from the ESPAC-3 trial should be considered in daily practice.
The combined results of 458 randomized pancreatic cancer patients from the ESPAC-1, ESPAC-1 plus, and early ESPAC-3 (version 1) trial results were also used to estimate the effectiveness of adjuvant fluorouracil/folinic acid compared to resection alone using meta-analysis (27). Median overall survival was 23.2 months with fluorouracil/folinic acid versus 16.8 months with resection alone, supporting adjuvant chemotherapy with fluorouracil/folinic acid in pancreatic cancer.
Between 2007 and 2010, adjuvant S-1, a fluorouracil prodrug with efficacy in Japanese patients, was compared to adjuvant gemcitabine in Japanese patients with resected pancreatic cancer within the Japanese JASPAC-01 trial (21). After a median follow-up of 40.6 and 39.2 months of patients in the gemcitabine group and those in the S-1 group, respectively, an interim analysis using data of the per-protocol population including 377 patients was performed. This interim analysis showed a hazard ratio (HR) for mortality of S-1 compared with gemcitabine of 0.57 (95% CI: 0.44–0.72, P-non-inferiority <0.0001, P<0.0001 for superiority). Following the recommendation from the independent data and safety monitoring committee, the trial was then discontinued because the pre-specified criteria for early discontinuation were met. The authors concluded that S-1 rather than gemcitabine should be the standard adjuvant treatment for resected pancreatic cancer in Japanese patients.
The CONKO-005 and CONKO-006 trials aimed to investigate the benefit of adjuvant gemcitabine combined with targeted therapies, namely erlotinib or sorafenib, versus gemcitabine monotherapy in patients with resected pancreatic cancer (22,23). In contrast to the palliative situation, however, neither the addition of erlotinib to gemcitabine nor the addition of sorafenib did result in improved survival for patients.
Current standards
The results from the ESPAC-4 trial, showing a significant survival benefit in patients with resected pancreatic cancer receiving adjuvant combination chemotherapy with gemcitabine and capecitabine compared to gemcitabine monotherapy, build the basis for today’s standard of care in the adjuvant setting (Figure 2) (24). Within the multicenter randomized controlled ESPAC-4 trial a total of 730 patients undergoing upfront resection for pancreatic cancer were randomized to 6 cycles of adjuvant chemotherapy with gemcitabine ± oral capecitabine. The median overall survival for patients in the gemcitabine plus capecitabine group was 28.0 months compared to 25.5 months in the gemcitabine only group. There was also a considerably higher 5-year survival rate in the couplet therapy group (28.8% versus 16.3%). Grade 3/4 toxicities were similar in both treatment arms and patients tolerated both regimens quite well. Based on these data, 6 months of adjuvant chemotherapy are currently recommended for patients with resected pancreatic cancer, preferably with combination of gemcitabine and capecitabine, and either with gemcitabine monotherapy or fluorouracil/folinic acid in case of concerns for toxicity or tolerance (28).
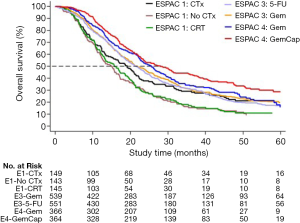
More recently, the results from the multicenter, randomized PRODIGE24/CCTGPA.6 French-Canadian trial comparing adjuvant mFOLFIRINOX against gemcitabine have shown unique median survival times of 54.4 months in the mFOLFIRINOX group compared to 35.0 months in the gemcitabine monotherapy group (5). In this trial, 493 patients with macroscopically resected pancreatic cancer, less than 80 years old, and ECOG performance status of 0 or 1, were randomized to either mFOLFIRINOX given every 2 weeks, or gemcitabine monotherapy given every 4 weeks, for 24 weeks. Grade 3/4 toxicities occurred in 75.9% of patients receiving mFOLFIRINOX compared to 52.9% of those receiving gemcitabine. Considering that adjuvant mFOLFIRINOX can achieve the longest overall survival yet reported for patients with resected pancreatic cancer, this regimen should be offered to selected patients with good performance status following upfront resection for pancreatic cancer.
A press release by Celgene on 12 March 2019 reported on the Celgene-sponsored, pivotal, randomized controlled phase 3 APACT® trial (NCT01964430) evaluating the investigational use of nab-paclitaxel (ABRAXANE) in combination with gemcitabine as adjuvant treatment following surgical resection in patients with pancreatic cancer (29). Compared to patients randomized to gemcitabine monotherapy, the patients randomized to gemcitabine-nab-paclitaxel did not achieve the primary endpoint of significant improvement in disease-free survival, as evaluated by independent radiological review. Celgene reported that overall survival, a secondary endpoint of the study, was improved, reaching “nominal statistical significance”, with ABRAXANE in combination with gemcitabine compared to gemcitabine alone, but no further details were provided (29). The USA Federal Drugs Administration does not approve ABRAXANE for the adjuvant treatment of pancreatic cancer.
Future perspectives
Despite advances in adjuvant chemotherapy regimen and associated improvements in postsurgical survival times, there is still a large proportion of patients with poor tumor response to the applied chemotherapy protocol while suffering from side effects. One major improvement in survival might be achievable by stratifying patients to current chemotherapy regimens based on treatment specific signatures, which need to be further developed and validated before using them in daily practice within a clinically relevant time frame (30). In addition, direct functional response testing of personalized models of human pancreatic cancer such as patient derived tumor organoids might be a beneficial approach to predict individual treatment responses. Specific prospective clinical trial designs are needed to investigate the potential oncological benefit of such personalized approaches compared to the standard therapies, although the response rates are sorrowfully poor in the vast majority of patients (31).
Tumor cell dissemination as a result of tumor manipulation during surgery may increase the risk for early tumor relapse and metastatic tumor spread in patients undergoing upfront resection. The use of intraoperative chemotherapy seems therefore reasonable and is currently investigated in the prospective combiCaRe trial (DRKS00015766). In addition, with the increasing use of neoadjuvant treatment strategies especially in patients with borderline and locally advanced pancreatic cancer, extend and value of adjuvant treatment in these situations need to be investigated. Results from ongoing trials such as the randomized ESPAC-5F feasibility trial (ISRCTN89500674) will build the basis for future trials on the use of neoadjuvant therapies compared to upfront surgery in patients with borderline resectable pancreatic cancer.
Summary
Even though the prognosis of pancreatic cancer patients is still dismal, the past few decades have seen considerable improvements in postsurgical survival times. Pancreatic resections have become safe procedures with mortality rates below 5% in specialized institutions even when extended tumors are resected. At the same time, enormous progress has been made in evidence-based development of adjuvant chemotherapy protocols which enable the chance for long-term survival. With the development of personalized approaches and the investigation of neoadjuvant and intraoperative treatment strategies further improvements might be achievable in the near future. Extend and value of adjuvant therapy following neoadjuvant or intraoperative treatment and resection also need to be defined.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Neoptolemos JP, Kleeff J, Michl P, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol 2018;15:333-48. [Crossref] [PubMed]
- Hackert T. Surgery for Pancreatic Cancer after neoadjuvant treatment. Ann Gastroenterol Surg 2018;2:413-8. [Crossref] [PubMed]
- Groot VP, Rezaee N, Wu W, et al. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg 2018;267:936-45. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Klaiber U, Leonhardt CS, Strobel O, et al. Neoadjuvant and adjuvant chemotherapy in pancreatic cancer. Langenbecks Arch Surg 2018;403:917-32. [Crossref] [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [Crossref] [PubMed]
- Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer 1987;59:2006-10. [Crossref] [PubMed]
- Bakkevold KE, Arnesjo B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater--results of a controlled, prospective, randomised multicentre study. Eur J Cancer 1993;29A:698-703. [Crossref] [PubMed]
- Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002;95:1685-95. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82. [Crossref] [PubMed]
- Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg 2007;246:734-40. [Crossref] [PubMed]
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-85. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer 2009;101:908-15. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol 2011;18:1319-26. [Crossref] [PubMed]
- Schmidt J, Abel U, Debus J, et al. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol 2012;30:4077-83. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [Crossref] [PubMed]
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248-57. [Crossref] [PubMed]
- Sinn M, Bahra M, Liersch T, et al. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol 2017;35:3330-7. [Crossref] [PubMed]
- Sinn M, Liersch T, Gellert K, et al. LBA18CONKO-006: A Randomized Double-Blinded Phase IIB-Study of Adjuvant Therapy with Gemcitabine + Sorafenib/Placebo for Patients with R1-Resection of Pancreatic Cancer. Ann Oncol 2014;25:mdu438.18.
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Twombly R. Adjuvant chemoradiation for pancreatic cancer: few good data, much debate. J Natl Cancer Inst 2008;100:1670-71. [Crossref] [PubMed]
- Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol 2014;32:504-12. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Tudur Smith C, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer 2009;100:246-50. [Crossref] [PubMed]
- Khorana AA, Mangu PB, Katz MHG. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract 2017;13:388-91. [Crossref] [PubMed]
- Celgene Provides Update on ABRAXANE® Combination Therapy in the Treatment of Metastatic Triple-Negative Breast Cancer and Pancreatic Cancer. Available online: . Released on March 12, 2019. Accessed on March 21, 2019.https://ir.celgene.com/press-releases/press-release-details/2019/Celgene-Provides-Update-on-ABRAXANE-Combination-Therapy-in-the-Treatment-of-Metastatic-Triple-Negative-Breast-Cancer-and-Pancreatic-Cancer/default.aspx
- Tiriac H, Belleau P, Engle DD, et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov 2018;8:1112-29. [Crossref] [PubMed]
- Marquart J, Chen EY, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Benefit From Genome-Driven Oncology. JAMA Oncol 2018;4:1093-8. [Crossref] [PubMed]
Cite this article as: Klaiber U, Hackert T, Neoptolemos JP. Adjuvant treatment for pancreatic cancer. Transl Gastroenterol Hepatol 2019;4:27.


