
Novel Barrett’s esophagus screening assays based on swallowable devices: will they change the game?
The incidence of several cancers, including colorectal cancer, has plateaued or even declined in recent years (1). Unfortunately, we have not seen a similar decrease in the incidence of esophageal adenocarcinoma (EAC). If anything, modeling studies estimate that deaths from EAC will continue to increase until 2030, eventually leading to nearly twice the number of deaths seen during the previous 20 years (2). This grim forecast is a consequence of EAC being challenging to treat as it is typically diagnosed in later stages, when cure by surgical resection or multimodal therapy is no longer possible (3). For this reason, improved early detection of EAC or its precursor lesion, Barrett’s esophagus (BE), is one strategy that has been proposed to reduce EAC mortality.
It is accepted that the vast majority of EAC is derived from BE, which is metaplastic epithelium in the esophagus that presumably arises to protect the esophagus against an ongoing barrage of gastric contents, including hydrochloric and bile acids and other damaging substances (4). BE has been recognized for two decades to be associated with a risk for EAC. In landmark studies in the late 1990s, Haggitt, Reid, and others demonstrated that non-dysplastic BE can progress via a series of histologic steps: first to BE with low-grade dysplasia, then to BE with high-grade dysplasia, and ultimately to EAC (5,6). Progression from BE to EAC only occurs in a minority of individuals, with the annual risk of EAC developing from BE currently estimated to be 0.11–0.33% (7).
BE is currently diagnosed in persons undergoing upper endoscopy for a variety of reasons, including in those with concerning symptoms (diagnostic endoscopy) or in people at increased risk for BE secondary to advanced age, male gender, and obesity (screening endoscopy). At present, the diagnosis of BE relies upon both its classic endoscopic appearance and histopathologic confirmation of intestinal metaplasia in esophageal biopsies taken during the endoscopy (7). Prevalence estimates of BE in the population are difficult to calculate because it is an asymptomatic condition but are thought to be roughly 15% of individuals with gastroesophageal reflux disease (GERD) and 1–2% of the general adult population (8). The impact of a diagnosis of BE is that periodic endoscopic surveillance is recommended, which can ultimately improve EAC survival by identifying earlier stage EAC. However, >90% of all EAC patients are unaware they had BE prior to their cancer diagnosis and are found with advanced-stage disease (9). Thus, it seems clear that we need to identify a greater proportion of the population with BE if we are to meaningfully reduce EAC deaths.
A major barrier to the identification of people with BE is that upper gastrointestinal (GI) tract endoscopy is the only screening test available. Although safe and accurate, endoscopy is an inconvenient and expensive screening test, which has led to controversy regarding the population health value of BE screening programs (4). This has led to intense interest in the development of an inexpensive, safe and accurate BE screening test that is acceptable to patients. To this end, a variety of techniques are being developed that are less costly and less invasive than upper endoscopy and that could be deployed on a population level. The most mature and promising of these emerging assays are based on swallowable balloons or capsules that are tethered to a string or small tube that remains outside the patient’s mouth. After being swallowed, the string is used to retrieve the device and collect cellular material from the esophagus for analysis. Of the current devices, the EsophaCap and Cytosponge were the first iterations of the swallowable cytology collection devices and consist of an expandable sponge (10,11). The JASSS balloon is a second-generation device consisting of a sheathed balloon, which can minimize cellular contamination from the upper esophagus and mouth (12). Over the last few years, several promising studies that use novel molecular or immunohistochemistry-based assays to detect BE from the material collected with these devices have been published (10-15) (Figure 1).

In a recent issue of Clinical Cancer Research, Wang et al. report on the accuracy of an assay using methylated DNA biomarkers collected with the EsophaCap swallowable device to diagnose BE (16). As noted above, the EsophaCap, which has also been evaluated in another recent study (11), consists of a polyurethane foam sphere attached to a filament. The sphere is compressed and packaged within a gelatin capsule. After it is swallowed, the capsule dissolves in the patient’s stomach after several minutes, and then the foam sphere is retrieved using the tethered string, capturing gastric and esophageal cells during its egress.
Wang et al evaluated 80 symptomatic patients referred for upper endoscopy at Johns Hopkins University from 2016–2018, dividing them into training (N=52) and test (N=28) sets. The EsophaCap device was swallowed and retrieved prior to endoscopy. DNA collected from the sponge after retrieval was analyzed using a relatively new technique called “Methylation on Beads” (MOB), which facilitates the detection of small amounts of methylated DNA. DNA was analyzed using quantitative methylation-specific PCR (qMSP) to determine the methylation levels of 8 previously selected candidate genes that have been shown to be methylated in BE and to possibly predict the risk of progression to EAC (17).
Importantly, the researchers were able to capture sufficient cellular material for their analyses using the EsophaCap device. The device was well tolerated and not associated with any complications, although the authors did not include participant feedback regarding their impressions of the device. Eighty/94 (85%) of participants were able to swallow the device.
In the training set, methylation levels of 5 of 8 candidate markers were higher in those with BE compared to controls (including CDKN2A/p16, HPP1, NELL1, TAC1, and AKAP12). The group analyzed the markers in several ways, but using a lasso regression model consisting of four biomarkers (CDKN2A/p16, NELL1, AKAP12, TAC1) plus age, they noted an area under the ROC curve (AUC) of 0.894, a sensitivity of 94.4% (95% CI: 71–99%), and a specificity of 62.2% (95% CI: 44.6–77.3%). Using the same markers and model in an independent test set, they achieved an AUC of 0.929, with a sensitivity of 78.6% (95% CI: 48.8–94.3%) and a specificity of 92.8% (95% CI: 64.1–99.6%).
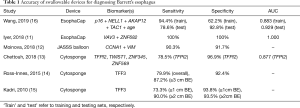
Overall, the accuracy for BE detection using the EsophaCap device coupled with methylated DNA markers in this small patient population was comparable to other previously published studies that evaluated other molecular markers for BE using non-endoscopic cell collection devices (Table 1). It is worth noting that in this study individuals who had BE were significantly older than controls and that age was included as a variable in the assay. It should also be noted that the patient population studied by Wang et al was highly enriched in BE cases, including 18/52 participants (34.6%) in the training set and 14/28 (50.0%) in the test set, proportions that are much higher than would be expected in the general population or even in a subgroup of individuals with GERD. It is likely that the high specificity values, particularly in the test set, reflect the high proportion of BE cases and that one might expect the specificity to drop in a screening population, as false positives would expect to increase with decreasing prevalence.
Full table
To put these results into context, for a screening test to be effective, it must be acceptable to the target population, since compliance is central to a screening test being successful in reducing cancer-related death. The authors of this study did not include information regarding tolerability or test preference, although a previous study evaluating the EsophaCap device showed this device was well tolerated, with low pain, choking, gagging, and anxiety scores. Additionally, most participants stated they would undergo the procedure again to screen for BE and preferred the capsule sponge to upper endoscopy to detect Barrett’s (11). Similar swallowable devices, including the Cytosponge, have been given “good” acceptability ratings by participants in other studies (10).
Other prerequisites for swallowable devices to be effective for BE screening include: (I) a high swallowing success rate; (II) consistent and robust esophageal sample collection rate; and (III) ease of use in the clinic. In general, these devices appear to be quite effective at capturing enough cellular material for analysis (there were no reported cases of inadequate sampling in the Wang study), but there is room for improvement in terms of swallowability. In the study by Wang et al., the EsophaCap was successfully swallowed 85% of the time, although Iyer et al. had a 98% success rate using the EsophaCap (11). The Cytosponge was successfully swallowed 91% of the time in prior studies (10), and the first-generation JASSS balloon was swallowed by 82% of the subjects (12). Based on success rates of other screening tests, a non-endoscopic device should be swallowable by at least 90% of the subjects and ideally ≥95% of individuals in a screening population to maximize the impact of the assay on screening. With further refinements to these devices, this should be readily achievable.
The impetus behind these and similar studies comes from the recognized need for safe, effective, inexpensive, and less invasive screening methods that can be applied to large populations. In addition to swallowable balloons and sponges, other devices, including a disposable transnasal video capsule (EG Scan) (18), a tethered optical coherence tomographic imaging capsule (19), detection of volatile organic compounds (VOC) (20), and blood-based detection of microRNAs (21), are being actively investigated as tools to detect BE. Outstanding updates on these emerging methods and the goals and pitfalls of implementing “mass screening” for BE have recently been published (22,23).
At the heart of the issue of mass screening for BE is defining the appropriate target screening population that will benefit from this intervention. Guidelines from professional GI societies are imprecise but suggest that clinicians consider screening for BE for individuals with chronic symptoms of GERD and additional risk factors for BE or EAC, including Caucasian race, age over 50 years, presence of central obesity, etc. (4,7). A population with GERD has a six-fold increased risk of EAC compared to a control population (23), however, a screening strategy based on GERD symptoms will miss the 40% of people with EAC who deny any prior reflux symptoms (24). A recently published model suggested that screening the 20% of the U.S. population with GERD would account for 52% of the EAC cancer cases (23,25). One of the reasons to limit the number of people who are currently eligible for screening is the cost and invasiveness of endoscopy-based screening. The adoption of screening programs based on swallowable devices would mitigate these factors and would be more readily available than endoscopy-based programs because a trained endoscopist is not needed.
A particularly timely and exciting aspect of these studies showing the feasibility of swallowable device-based molecular BE screening assays is the advent of effective endoscopic treatment therapies that can eradicate dysplastic Barrett’s and early-stage EAC without the need for esophagectomy. To realize the potential impact of these emerging BE biomarker assays, larger prospective trials in targeted populations, such as the ongoing BEST3 study with the Cytosponge and clinical trials supported by the BETRNET, EDRN, and GI SPORE mechanisms, are necessary to further establish that these devices are safe, accurate, easy to administer, and cost-effective. If these studies show that swallowable device-based methods are effective and more people are screened for BE, issues related to overdiagnosis and overtreatment will need to be addressed, as will the need for non-endoscopic surveillance methods that are inexpensive, accurate and safe. There are also ongoing studies to determine the potential for molecular assays that use the swallowable devices to be used for surveillance of high-grade dysplasia and early EAC. Ultimately, we will need to reach a consensus about how to implement the most effective BE screening system if programmatic testing is to diminish the number of EAC cases predicted to come in the near future.
Acknowledgements
Funding: This material is the result of work supported in part by resources from the VA Puget Sound Heath Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Support for this work was provided by National Institutes of Health (NIH) National Cancer Institute (NCI) RO1CA115513, P30CA15704, UO1CA152756, U54CA143862, and P01CA077852 (WM Grady).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124:2785-800. [Crossref] [PubMed]
- Kong CY, Kroep S, Curtius K, et al. Exploring the recent trend in esophageal adenocarcinoma incidence and mortality using comparative simulation modeling. Cancer Epidemiol Biomarkers Prev 2014;23:997-1006. [Crossref] [PubMed]
- Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013;119:1149-58. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. American College of G. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol 2016;111:30-50. [Crossref] [PubMed]
- Reid BJ, Barrett MT, Galipeau PC, et al. Barrett's esophagus: ordering the events that lead to cancer. Eur J Cancer Prev 1996;5 Suppl 2:57-65. [Crossref] [PubMed]
- Haggitt RC. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol 1994;25:982-93. [Crossref] [PubMed]
- Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011;140:1084-91. [Crossref] [PubMed]
- Thrift AP. Barrett's Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Dig Dis Sci 2018;63:1988-96. [Crossref] [PubMed]
- Bhat SK, McManus DT, Coleman HG, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett's oesophagus: a population-based study. Gut 2015;64:20-5. [Crossref] [PubMed]
- Januszewicz W, Tan WK, Lehovsky K, et al. Safety and Acceptability of Esophageal Cytosponge Cell Collection Device in a Pooled Analysis of Data From Individual Patients. Clin Gastroenterol Hepatol 2019;17:647-56.e1. [Crossref] [PubMed]
- Iyer PG, Taylor WR, Johnson ML, et al. Highly Discriminant Methylated DNA Markers for the Non-endoscopic Detection of Barrett's Esophagus. Am J Gastroenterol 2018;113:1156-66. [Crossref] [PubMed]
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett's esophagus. Sci Transl Med 2018. [Crossref] [PubMed]
- Chettouh H, Mowforth O, Galeano-Dalmau N, et al. Methylation panel is a diagnostic biomarker for Barrett's oesophagus in endoscopic biopsies and non-endoscopic cytology specimens. Gut 2018;67:1942-9. [Crossref] [PubMed]
- Ross-Innes CS, Debiram-Beecham I, O'Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS Med 2015;12:e1001780. [Crossref] [PubMed]
- Kadri SR, Lao-Sirieix P, O'Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ 2010;341:c4372. [Crossref] [PubMed]
- Wang Z, Kambhampati S, Cheng Y, et al. Methylation Biomarker Panel Performance in EsophaCap Cytology Samples for Diagnosing Barrett's Esophagus: A Prospective Validation Study. Clin Cancer Res 2019;25:2127-35. [Crossref] [PubMed]
- Jin Z, Cheng Y, Gu W, et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett's esophagus. Cancer Res 2009;69:4112-5. [Crossref] [PubMed]
- Sami SS, Iyer PG, Pophali P, J, et al. Acceptability, Accuracy, and Safety of Disposable Transnasal Capsule Endoscopy for Barrett's Esophagus Screening. Clin Gastroenterol Hepatol 2019;17:638-46.e1. [Crossref] [PubMed]
- Bhardwaj A, Hollenbeak CS, Pooran N, et al. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett's esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol 2009;104:1533-9. [Crossref] [PubMed]
- Chan DK, Zakko L, Visrodia KH, et al. Breath Testing for Barrett's Esophagus Using Exhaled Volatile Organic Compound Profiling With an Electronic Nose Device. Gastroenterology 2017;152:24-6. [Crossref] [PubMed]
- Mallick R, Patnaik SK, Wani S, et al. A Systematic Review of Esophageal MicroRNA Markers for Diagnosis and Monitoring of Barrett's Esophagus. Dig Dis Sci 2016;61:1039-50. [Crossref] [PubMed]
- Thota PN, Chak A. Is Mass Screening for Barrett's Esophagus a Myth or Reality? Clin Gastroenterol Hepatol 2019;17:610-12. [Crossref] [PubMed]
- O'Donovan M, Fitzgerald RC. Screening for Barrett's Esophagus: Are New High-Volume Methods Feasible? Dig Dis Sci 2018;63:2105-14. [Crossref] [PubMed]
- Chak A, Faulx A, Eng C, et al. Gastroesophageal reflux symptoms in patients with adenocarcinoma of the esophagus or cardia. Cancer 2006;107:2160-6. [Crossref] [PubMed]
- Vaughan TL, Fitzgerald RC. Precision Prevention of Oesophageal Adenocarcinoma. Nat Rev Gastroenterol Hepatol 2015;12:243-8. [Crossref] [PubMed]
Cite this article as: Kaz AM, Grady WM. Novel Barrett’s esophagus screening assays based on swallowable devices: will they change the game? Transl Gastroenterol Hepatol 2019;4:25.


