
Endoscopic management of pancreatic fluid collections
Acute pancreatitis is an acute inflammatory process of the pancreas which presents with severe epigastric abdominal pain along with at least a 3-fold increase in the serum lipase. It is one of the most common gastroenterology discharge diagnoses and costs an estimated $2.6 billion annually (1). Acute pancreatitis is classified as either interstitial edematous pancreatitis or necrotizing pancreatitis. Pancreatic fluid collections (PFCs) are common complications of acute pancreatitis, and are characterized based on the revised Atlanta classification (Table 1). There are four subtypes of PFCs: acute peripancreatic fluid collections, acute necrotic collections, pseudocysts, and walled-off necrosis (WON). These collections are differentiated based on duration (less than or greater than 4 weeks from onset of acute pancreatitis) and presence or absence of necrosis. Interstitial edematous pancreatitis can lead to acute peripancreatic fluid collections (<4 weeks) or pancreatic pseudocysts (>4 weeks). Necrotizing pancreatitis can lead to acute necrotic collections (<4 weeks) and WON (>4 weeks) (2).
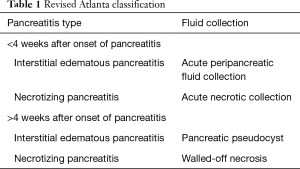
Full table
A 2018 study evaluated the outcomes of acute pancreatic and peripancreatic collections that occur in patients with acute pancreatitis. The study demonstrated that the majority of patients that present with interstitial edematous pancreatitis will not develop pseudocysts. This was in contrast to patients who initially present with necrotizing pancreatitis. Of these patients, 93.4% developed acute necrotic collections. Of those that survived, 70% of the acute necrotic collections developed into walled off necrosis (3).
The management of peripancreatic fluid collections has evolved with time. Most acute peripancreatic fluid collections or acute necrotic collections do not require any intervention as they may improve with conservative management. Conservative management usually entails antibiotics and nutritional support. Asymptomatic pseudocysts and WON also do not require any intervention as they may resolve on their own. If a patient is symptomatic from their pancreatic fluid collection, then it may need to be drained. Symptoms may include persistent abdominal pain, fevers, early satiety, nausea and vomiting, inability to tolerate diet, and concern for infection.
Draining symptomatic collections can be accomplished surgically, percutaneously, or endoscopically. Many studies have looked at the optimal approach for management. Van Santvoort et al. in 2010 and Rasch et al. in 2016 demonstrated that a minimally invasive step-up approach decreased mortality and complications when compared to open necrosectomy (4,5). Since then, a minimally invasive approach has been favored for management of pancreatic fluid collections. Bakker et al. conducted a randomized trial investigating endoscopic vs surgical necrosectomy for infected necrotizing pancreatitis (6). They demonstrated the overall pro-inflammatory response, new-onset organ failure, and complication rate was significantly lower in the endoscopic group. Akshintala et al. compared endoscopic vs percutaneous drainage for symptomatic pseudocysts. Although both groups achieved similar clinical success, the percutaneous drainage group had significantly higher rates of re-intervention, increased number of follow-up imaging studies, and longer length of hospital stay (7). More recently, a multicenter randomized trial evaluated endoscopic versus a surgical step-up approach to necrotizing pancreatitis. The endoscopic approach consisted of endoscopic transluminal drainage with nasocystic tube placement followed by direct endoscopic necrosectomy if transluminal drainage alone did not lead to considerable clinical improvement. The surgical step-up approach consisted of percutaneous catheter drainage followed by video-assisted retroperitoneal debridement (VARD), if necessary. This study demonstrated that the endoscopic approach was not superior to the surgical step-up approach in terms of major complications or death. The endoscopic step-up approach did yield a lower hospital length of stay and lower rate of pancreatic fistulae (8).
Endoscopic drainage of pancreatic collections has also evolved with time. Prior to the widespread use of linear echoendoscopes, pseudocysts or walled off necrosis collections were drained endoscopically based on luminal bulging (9). Endoscopic drainage therefore could only be carried out if a bulge was seen within the lumen of the GI tract (10). Endosonography now allows visualization of the fluid collection prior to drainage and does not require the collection to cause a deformity in the stomach or duodenum. This is especially helpful for smaller collections and collections near the tail of the pancreas. EUS-guided drainage of these collections has also changed with the advent of newer, easy-to-use devices. Although these new devices may be easier to use, it is important to understand and be able to perform a traditional EUS-guided pseudocyst or WON drainage as cost and availability of new devices may not be universal.
Once the pseudocyst or WON is located endosonographically, Doppler flow should be utilized to ensure there are no large intervening blood vessels between the lumen and the fluid collection. An optimal window should also be located to ensure the distance between the lumen and fluid collection is not large. Usually the distance between the collection and the lumen should be less than 10 mm. A 19-gauge needle is then used to puncture through the gastric or duodenal wall and into the fluid collection. Material from the collection can then be aspirated and sent for culture if there is suspicion of infection. A long wire, usually hydrophilic 0.035-inch guidewire, is inserted through the 19-gauge needle and allowed to coil in the fluid collection which is confirmed by fluoroscopy. The needle is then removed while keeping the wire in place. Next, a fistula needs to be created. The tract between the stomach and fluid collection can be dilated in a graded fashion using endoscopic cannulas and catheter dilators (11,12). In order to be able to do this, the catheter must be “in-line” with the wire to facilitate easy passage into the fluid collection. This may not always be possible. Therefore, a fistula tract can be created using a cautery device, such as a needle-knife or cystotome (13,14). The tract can then be dilated with a 6 or 8 mm balloon dilator. If dealing with a pseudocyst, plastic stents or a metal stent can be placed into the collection. There is data suggesting draining pseudocysts with plastic stents is comparable to metal biliary stents and is more cost-effective, while there is also some data suggesting there are less complications associated with using a fully covered self-expanding metal stent (FCSEMS) as opposed to plastic stents (15,16). Dilation of the tract allows the passage of a variety of different stents. Even fully covered esophageal stents have been placed into pancreatic fluid collections to aid in drainage (17). The FCSEMS can then be anchored in place by placing a plastic double pigtail stent through the FCSEMS (18). This helps prevent migration.
Newer lumen-apposing metal stents (LAMS), such as the AXIOS stent (Boston Scientific, Marlborough, MA United States), Nagi stent (Taewoong Medical Co, Ilsan, South Korea), and Niti-S SPAXUS stent (TaeWoong Medical Co., Ltd., Ilsan, South Korea) have revolutionized drainage of pancreatic fluid collections. Specifically, these stents help with direct endoscopic necrosectomy. These stents are fully-covered, dumbbell shaped stents that decrease the risk of migration and have a larger diameter which facilitates drainage and allows easier access for endoscopic necrosectomy (19,20) (Figure 1). This LAMS can be placed similar to other FCSEMS. After dilation of the fistula tract, this stent is advanced over the wire into the fluid collection. The internal flange of the LAMS is then deployed under endosonographic visualization. Once open, it is pulled to the wall of the fluid collection to slightly deform the shape, and then the proximal flange is deployed into the GI tract lumen. Once fully deployed, the waist can be dilated to the official diameter of the LAMS (21). These stents are available in the United States in 10, 15, and 20 mm diameter sizes.
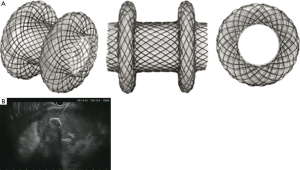
The LAMS is now available in an electrocautery-enhanced version (HOT AXIOS) as well. This helps eliminate multiple steps in EUS-guided pancreatic fluid collection drainage. The electrocautery-enhanced LAMS delivery system has an electrocautery wire at the distal tip of the delivery system. This allows direct access to a pancreatic fluid collection without the need to first puncture with a needle, pass a wire, or dilate the fistula tract. As a safety net, a wire can be passed through the delivery system. Once the delivery system has cauterized into the fluid collection, cautery is disabled, and the LAMS is deployed as mentioned earlier. This drastically decreases the amount of time required to perform the procedure and makes the device more user-friendly (22).
If draining a pseudocyst, the stents are left in place until the collection has resolved which can occur relatively quickly. Therefore plastic stents, FCSEMS, or LAMS can be removed within a few weeks. For WON though, the stent may need to be left in longer and direct endoscopic necrosectomy may need to be performed. With the advent of the LAMS, plastic stents and FCSEMS are no longer the primary stent used for drainage of WON. When comparing LAMS to biliary FCSEMS, the number of procedures required for resolution of WON is significantly lower with LAMS than with FCSEMS (23). The design of the LAMS prevents migration and facilitates necrosectomy. An adult gastroscope or therapeutic endoscope can easily be advanced into a WON through a LAMS.
There is currently no consensus guideline on how often direct endoscopic necrosectomy needs to be performed. There are differing opinions on the interval between each necrosectomy session and what tools should be used. Necrosis can be thick, adherent, and of a mud-like consistency (Figure 2). Aggressive normal saline lavage can help break up some of the necrotic tissue. Necrosectomy is then performed using snares, retrieval nets, stone-retrieval baskets, and rat-toothed forceps. The tissue is grasped and the pulled through the LAMS and discarded into the GI tract lumen (24). This can be a long, arduous process and the endoscopist and the patient need to be ready for this task. It is also important to note that many patients will require multiple necrosectomy sessions. The number of necrosectomy sessions varies based on the size of the WON. Clinical success of direct endoscopic necrosectomy ranges from 75% to 90% (24,25).
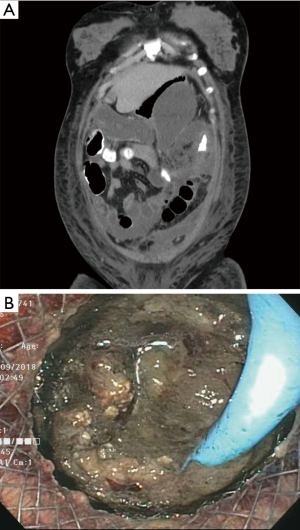
The tools used for endoscopic necrosectomy are not specifically designed for that use, making necrosectomy very difficult. Other methods to facilitate necrosectomy have been postulated. Lavaging the WON with 3% hydrogen peroxide, usually diluted 1:5 or 1:10, has been shown to help facilitate tissue dislodgement and debridement (26). Discontinuing proton pump inhibitor therapy may encourage auto-digestion of the necrotic tissue by normal physiologic gastric acid production (27). Placement of a nasocystic tube to allow the WON to be irrigated with normal saline has been shown to decrease the rate of stent occlusion and may decrease time to resolution (28). The multiple transluminal gateway technique has also been described, where multiple transmural tracts are created between the WON and the GI tract lumen using EUS. A nasocystic tube is then placed into one of the tracts and normal saline is flushed through the collection. This allows necrotic debris to drain from multiple areas of the collection (29).
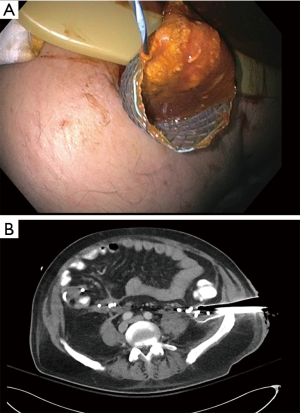
For large necrotic collection, especially those that extend into the pelvis, it may not be possible to perform endoscopic necrosectomy. In these situations, percutaneous drains can be placed by interventional radiology to aid in drainage. Percutaneous drains are often not large enough to facilitate drainage of necrotic tissue. In those situations, the percutaneous drain can be used as an access point as long as the collection is near the skin. Navarrette et al., describes using this as a way to access the pelvic portion of a WON and perform necrosectomy. This is done by placing a wire through the percutaneous drain and into the collection. The percutaneous drain is then removed with the wire in place. A fully covered esophageal stent is then placed percutaneously over the wire into the collection using fluoroscopy with the distal end of the stent protruding from the skin (Figure 3). The stent is sutured to the abdominal wall to prevent migration and an endoscope is inserted through the esophageal stent into the collection, and necrosectomy is then performed (30).
It is important to assess the integrity of the pancreatic duct either by MRCP or ERCP. A pancreatic duct disruption/leak or stricture can result in failure of a fluid collection to resolve. This can also lead to recurrence of a pancreatic fluid collection after transmural drainage. Trevino et al. have shown that transpupillary stenting leads to successful resolution of pancreatic duct disruptions, especially in the cases of a partial disruption (31). Shrode et al. confirmed these findings and showed that transpupillary stenting in the setting of a complete pancreatic duct disruption likely was not beneficial (32). A multicenter study by Yang et al. stated there was no benefit of transpupillary drainage in patients who underwent EUS-guided transmural drainage of pseudocysts (33). These findings are a bit contentious as there was no subgroup analysis of those patients who had transpupillary stenting across an area of confirmed pancreatic duct leak. It is important for a pancreatic stent to bridge a disruption or leak when possible (34).
Endoscopic drainage of pancreatic fluid collections is relatively safe, but complications can occur. Major adverse events such as perforation, bleeding, stent migration/occlusion, and infection are not common but can occur. Overall complication rates vary anywhere from 4% to 21% (35,36). It is important to understand the risk of these procedures and to convey these risks to the patients prior to proceeding with endoscopic drainage.
EUS-guided transmural drainage of pancreatic fluid collections is an advanced endoscopic procedure which requires adequate knowledge of EUS. Training in therapeutic EUS is not universal. The American Society for Gastrointestinal Endoscopy (ASGE) in 2001, recommended a total of 150 EUS cases, of which 50 are EUS-FNAs be required before competency could be assessed for endoscopic ultrasound (37). More recently, the ASGE has recommended a total of 225 EUS cases before competency can be assessed (38). The European Society of Gastrointestinal Endoscopy (ESGE) recommends 20–30 supervised EUS-FNAs of non-pancreatic and pancreatic lesions, respectively, before competency for EUS-FNA can be assessed (39). Obviously, this requires competence in EUS with a linear echoendoscope. Wani et al. has demonstrated that there is great variability in achieving competency in EUS among advanced endoscopy trainees. In his study, only 2 of 5 advanced endoscopy trainees achieved competency at 255 and 295 cases respectively. Two others showed a trend toward acceptable performance, and one demonstrated a need for ongoing training (40). This demonstrates that many advanced endoscopy trainees may not be competent to perform pancreatic fluid collection drainage and necrosectomy as they may not be proficient in diagnostic and basic therapeutic EUS. A multicenter study evaluating learning curves and competence in EUS and ERCP, demonstrated that out of 17 advanced endoscopy trainees from high volume academic centers, 82% (14) achieved technical competence and 76% (13) achieved cognitive competence in EUS at the end of training. The median number of EUS procedures was 300. The majority (~85%) of these trainees felt comfortable performing pseudocyst drainage based on a post-study questionnaire (41). Varadarajulu et al. demonstrated that as a high-volume advanced endoscopist (over 500 EUS procedures yearly), technical proficiency for performing cystgastrostomy took 25 cases (12). This was prior to the advent of the LAMS deployment systems. The number of cases needed to achieve technical proficiency in performing cystgastrostomy may be lower now given the ease of use of LAMS but still requires competence in diagnostic and basic therapeutic EUS.
The treatment of pancreatic fluid collections has evolved drastically over the last 10 years. New equipment and techniques have helped advanced endoscopists deal with these collections in a minimally invasive manner, replacing the more morbid surgical interventions. The available literature shows that endoscopic drainage is safe and efficacious. There is currently no consensus on the optimal approach or timing of endoscopic drainage and necrosectomy. The learning curve for performing these procedures is not known but a comprehensive knowledge of diagnostic and therapeutic EUS is necessary. It is important that patients with symptomatic pancreatic fluid collections be managed at centers that perform a high volume of endoscopic drainage procedures. It is also important to have highly-skilled and knowledgeable interventional radiologists and pancreatic surgeons as some of these collections may require a multidisciplinary approach for management.
Acknowledgements
None.
Footnote
Conflicts of Interest: H Shahid is a consultant for US Endoscopy.
References
- Fagenholz PJ, Fernandez-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas 2007;35:302-7. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis 2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Manrai M, Kochhar R, Gupta V, et al. Outcome of Acute Pancreatic and Peripancreatic Collections Occurring in Patients With Acute Pancreatitis. Ann Surg 2018;267:357-63. [Crossref] [PubMed]
- van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491-502. [Crossref] [PubMed]
- Rasch S, Phillip V, Reichel S, et al. Open Surgical versus Minimal Invasive Necrosectomy of the Pancreas-A Retrospective Multicenter Analysis of the German Pancreatitis Study Group. PLoS One 2016;11:e0163651. [Crossref] [PubMed]
- Bakker OJ, van Santvoort HC, van Brunschot S, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012;307:1053-61. [Crossref] [PubMed]
- Akshintala VS, Saxena P, Zaheer A, et al. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc 2014;79:921-8. [Crossref] [PubMed]
- van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet 2018;391:51-8. [Crossref] [PubMed]
- Park DH, Lee SS, Moon SH, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy 2009;41:842-8. [Crossref] [PubMed]
- Baron TH. Drainage of pancreatic fluid collections: is EUS really necessary?. Gastrointest Endosc 2007;66:1123-5. [Crossref] [PubMed]
- Ahlawat SK, Charabaty-Pishvaian A, Jackson PG, et al. Single-step EUS-guided pancreatic pseudocyst drainage using a large channel linear array echoendoscope and cystotome: results in 11 patients. JOP 2006;7:616-24. [PubMed]
- Varadarajulu S, Tamhane A, Blakely J. Graded dilation technique for EUS-guided drainage of peripancreatic fluid collections: an assessment of outcomes and complications and technical proficiency (with video). Gastrointest Endosc 2008;68:656-66. [Crossref] [PubMed]
- Azar RR, Oh YS, Janec EM, et al. Wire-guided pancreatic pseudocyst drainage by using a modified needle knife and therapeutic echoendoscope. Gastrointest Endosc 2006;63:688-92. [Crossref] [PubMed]
- Kitamura K, Yamamiya A, Ishii Y, et al. Electrocautery vs non-electrocautery dilation catheters in endoscopic ultrasonography-guided pancreatic fluid collection drainage. World J Gastrointest Endosc 2016;8:458-65. [Crossref] [PubMed]
- Bang JY, Varadarajulu S. Metal versus Plastic Stent for Transmural Drainage of Pancreatic Fluid Collections. Clin Endosc 2013;46:500-2. [Crossref] [PubMed]
- Sharaiha RZ, DeFilippis EM, Kedia P, et al. Metal versus plastic for pancreatic pseudocyst drainage: Clinical outcomes and success. Gastrointest Endosc 2015;82:822-7. [Crossref] [PubMed]
- Sarkaria S, Sethi A, Rondon C, et al. Pancreatic necrosectomy using covered esophageal stents: A novel approach. J Clin Gastroenterol 2014;48:145-52. [Crossref] [PubMed]
- Penn DE, Draganov PV, Wagh MS, et al. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 2012;76:679-84. [Crossref] [PubMed]
- Sharaiha RZ, Tyberg A, Khashab MA, et al. Endoscopic Therapy With Lumen-apposing Metal Stents Is Safe and Effective for Patients With Pancreatic Walled-off Necrosis. Clin Gastroenterol Hepatol 2016;14:1797-803. [Crossref] [PubMed]
- Siddiqui AA, Adler DG, Nieto J, et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest Endosc 2016;83:699-707. [Crossref] [PubMed]
- Shah RJ, Shah JN, Waxman I, et al. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol 2015;13:747-52. [Crossref] [PubMed]
- Rinninella E, Kunda R, Dollhopf M, et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: a large retrospective study (with video). Gastrointest Endosc 2015;82:1039-46. [Crossref] [PubMed]
- Siddiqui AA, Kowalski TE, Loren DE, et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: Clinical outcomes and success. Gastrointest Endosc 2017;85:758-65. [Crossref] [PubMed]
- Gardner TB, Chahal P, Papachristou GI, et al. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc 2009;69:1085-94. [Crossref] [PubMed]
- Seifert H, Biermer M, Schmitt W, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut 2009;58:1260-6. [Crossref] [PubMed]
- Siddiqui AA, Easler J, Strongin A, et al. Hydrogen peroxide-assisted endoscopic necrosectomy for walled-off pancreatic necrosis: a dual center pilot experience. Dig Dis Sci 2014;59:687-90. [Crossref] [PubMed]
- Thompson CC, Kumar N, Slattery J, et al. A Standardized Method for Endoscopic Necrosectomy Improves Complication and Mortality Rates. Pancreatology 2016;16:66-72. [Crossref] [PubMed]
- Siddiqui AA, Dewitt JM, Strongin A, et al. Outcomes of EUS-guided drainage of debris-containing pancreatic pseudocysts by using combined endoprosthesis and a nasocystic drain. Gastrointest Endosc 2013;78:589-95. [Crossref] [PubMed]
- Varadarajulu S, Phadnis MA, Christein JD, et al. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc 2011;74:74-80. [Crossref] [PubMed]
- Navarrete C, Castillo C, Caracci M, et al. Wide percutaneous access to pancreatic necrosis with self-expandable stent: new application (with video). Gastrointest Endosc 2011;73:609-10. [Crossref] [PubMed]
- Trevino JM, Tamhane A, Varadarajulu S. Successful stenting in ductal disruption favorably impacts treatment outcomes in patients undergoing transmural drainage of peripancreatic fluid collections. J Gastroenterol Hepatol 2010;25:526-31. [Crossref] [PubMed]
- Shrode CW, Macdonough P, Gaidhane M, et al. Multimodality endoscopic treatment of pancreatic duct disruption with stenting and pseudocyst drainage: how efficacious is it? Dig Liver Dis 2013;45:129-33. [Crossref] [PubMed]
- Yang D, Amin S, Gonzalez S, et al. Transpapillary drainage has no added benefit on treatment outcomes in patients undergoing EUS-guided transmural drainage of pancreatic pseudocysts: a large multicenter study. Gastrointest Endosc 2016;83:720-9. [Crossref] [PubMed]
- Tyberg A, Kahaleh M. Transpapillary drainage has a major benefit on treatment outcomes in patients undergoing EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 2016;83:1046-7. [Crossref] [PubMed]
- Lakhtakia S, Basha J, Talukdar R, et al. Endoscopic "step-up approach" using a dedicated biflanged metal stent reduces the need for direct necrosectomy in walled-off necrosis (with videos). Gastrointest Endosc 2017;85:1243-52. [Crossref] [PubMed]
- Vazquez-Sequeiros E, Baron TH, Pérez-Miranda M, et al. Evaluation of the short- and long-term effectiveness and safety of fully covered self-expandable metal stents for drainage of pancreatic fluid collections: results of a Spanish nationwide registry. Gastrointest Endosc 2016;84:450-7.e2. [Crossref] [PubMed]
- Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc 2001;54:811-4. [Crossref] [PubMed]
- ASGE Standards of Practice Committee, Faulx AL, Lightdale JR, et al. Guidelines for privileging, credentialing, and proctoring to perform GI endoscopy. Gastrointest Endosc 2017;85:273-81. [Crossref] [PubMed]
- Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy 2012;44:190-206. [Crossref] [PubMed]
- Wani S, Cote GA, Keswani R, et al. Learning curves for EUS by using cumulative sum analysis: implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest Endosc 2013;77:558-65. [Crossref] [PubMed]
- Wani S, Keswani R, Hall M, et al. A Prospective Multicenter Study Evaluating Learning Curves and Competence in Endoscopic Ultrasound and Endoscopic Retrograde Cholangiopancreatography Among Advanced Endoscopy Trainees: The Rapid Assessment of Trainee Endoscopy Skills Study. Clin Gastroenterol Hepatol 2017;15:1758-67.e11. [Crossref] [PubMed]
Cite this article as: Shahid H. Endoscopic management of pancreatic fluid collections. Transl Gastroenterol Hepatol 2019;4:15.


