
Banana may be forbidden after endoscopic variceal ligation: a case report
Introduction
Acute variceal hemorrhage (AVH) is a lethal consequence of portal hypertension in liver cirrhosis patients (1,2). The general management of AVH includes blood volume restitution, pharmacotherapy, endoscopic treatment, and transjugular intrahepatic portosystemic shunt (TIPS) (3,4). According to the current international consensus and guidelines, vasoconstrictors (i.e., somatostatin, octreotide, and terlipressin) plus endoscopic therapy [i.e., endoscopic variceal ligation (EVL), endoscopic sclerotherapy, and tissue adhesive injection] in combination with antibiotics (i.e., ceftriaxone and norfloxacin) should be used as the first-line choice of therapy (3,5). EVL is recommended as the preferred endoscopic treatment for esophageal varices (6). However, in clinical practice, there is no standard principle of dietary after EVL. There is no consensus regarding when to re-initiate the dining and which food can be fed.
Herein, we reported a case treated with EVL for AVH who self-assertively ate banana at the 5th postoperative day and developed acute upper gastrointestinal hemorrhage.
Case presentation
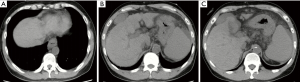
On May 24, 2018, a 41-year-old male was admitted to the Department of Emergency of our hospital due to hematemesis and melena for one day. At the Department of Emergency, he underwent abdominal computed tomography (CT) scans, which showed liver cirrhosis, splenomegaly, ascites, esophageal varices, and cholecystitis (Figure 1). Laboratory tests demonstrated that white blood cell (WBC) was 4.2×109/L [reference range: (3.5–9.5)×109/L], red blood cell (RBC) was 2.04×1012/L [reference range: (4.3–5.8)×1012/L], hemoglobin (HB) was 57 g/L (reference range: 130–175 g/L), platelet (PLT) was 58×109/L [reference range: (125–350)×109/L], total bilirubin (TBIL) was 13.5 µmol/L (reference range: 5.1–22.2 µmol/L), albumin (ALB) was 24.2 g/L (reference range: 40–55 g/L), prothrombin time (PT) was 19.0 seconds (reference range: 11.5–14.5 seconds), international normalized ratio (INR) was 1.6 (reference range: 0.9–1.1), and blood ammonia was 146 µmol/L (reference range: 9–54 µmol/L). He had Child-Pugh class B. He received intravenous infusion of esomeprazole 40 mg and somatostatin 6 mg. He developed melena again and then was transferred to our department. He had a history of arterial hypertension for 10 years and started to take oral propranolol 2 years ago. He was diagnosed with alcoholic liver cirrhosis in 2014; meanwhile, he was also diagnosed with hepatocellular carcinoma and was treated with transarterial embolization twice (in 2014 and 2015). He had a history of alcohol abuse for more than 20 years but abstained 2 years ago. At our department, he was initially treated with continuous intravenous infusion of terlipressin 2 mg per 12 hours and esomeprazole 80 mg per 12 hours, intravenous infusion of L-Ornithine L-Aspartate 10 g per day and ceftriaxone 1 g per day, and transfusion of packed red blood cell (PRBC) 2 units and fresh frozen plasma 210 mL.
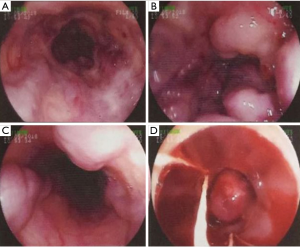
On May 25, 2018, an endoscopy showed several varices in the middle and lower parts of the esophagus with red color sign and visible thrombus (Figure 2). He underwent EVL. After the procedure, his attending physician told that he should be fast absolutely for at least 24 hours. At that time, esomeprazole was also discontinued. Transfusion of PRBC 2 units and fresh frozen plasma 160 mL were given.
On May 26, 2018, laboratory tests demonstrated that WBC was 3.7×109/L, RBC was 2.45×1012/L, HB was 70 g/L, PLT was 49×109/L. At that time, terlipressin was discontinued. He was also allowed to drink water and then gradually eat all-liquid food (i.e., rice soups and locus root starch). He was also given oral propranolol 20 mg bid.
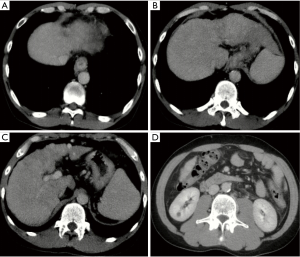
On the morning of May 29, 2018, contrast-enhanced CT scans demonstrated liver cirrhosis, splenomegaly, a cyst in right kidney, and no ascites (Figure 3). At 16 o’clock on May 29, 2018, he ate a banana and then presented with a sudden onset of abdominal discomfort and subsequent hematemesis with a fresh blood of 120 mL. His blood pressure dropped to 71/48 mmHg and blood oxygen saturation dropped to 86%. Urgent laboratory tests demonstrated that WBC was 5.9×109/L, RBC was 3.04×1012/L, HB was 85 g/L, PLT was 122×109/L, TBIL was 10.1 µmol/L, and ALB was 30.9 g/L. He received intravenous infusion of hydroxyethyl starch 500 mg and intravenous injection of terlipressin 1 mg. Then, he received a continuous intravenous infusion of terlipressin 2 mg per 6 hours and esomeprazole 80 mg per 12 hours. He intermittently had melena without hematemesis.
On May 30, 2018, he had melena twice again with a stool of about 300 mL. Laboratory tests demonstrated that WBC was 4.9×109/L, RBC was 2.45×1012/L, HB was 67 g/L, PLT was 98×109/L, PT was 17.5 seconds, and INR was 1.45. He received intravenous transfusion of fresh frozen plasma 160 mL. The dosage of terlipressin was reduced to 2 mg per 12 hours.
On June 2, 2018, hematemesis and/or melena had disappeared for more than 72 hours. Thus, terlipressin was discontinued. Laboratory tests demonstrated that WBC was 3.1×109/L, RBC was 2.39×1012/L, HB was 68 g/L, and PLT was 104×109/L. He was discharged on June 4, 2018 and continued to take oral esomeprazole 40 mg per day and oral propranolol 20 mg bid. Heart rate was about 70 beats per minutes and blood pressure was about 120/70 mmHg.
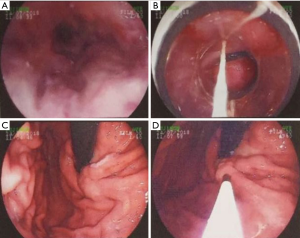
On July 23, 2018, he was stable and underwent follow-up endoscopic surveillance at our department. Laboratory tests demonstrated that WBC was 3.2×109/L, RBC was 3.34×1012/L, HB was 84 g/L, and PLT was 89×109/L. On July 24, 2018, he underwent EVL again in combination with tissue injection of gastric varices (Figure 4). Oral propranolol 20 mg bid was continued.
Discussion
Endoscopic examination should be performed within 12 hours for patients with liver cirrhosis and upper gastrointestinal bleeding (3). Currently, EVL is considered as the most effective choice of endoscopic treatment for esophageal varices, which has relatively less complications (7,8). EVL refers to the placement of rubber ligation bands on varices. The rubber ligation bands usually fall off within 5–7 days, leaving shallow ulcers which gradually heal and produce scars (9). Traditionally, it is recommended that absolute fasting should be given for 2 or 3 days after an EVL because early refeeding may cause elevated pressure and increased risk of variceal re-bleeding due to a shift in blood flow to the splanchnic circulation (10). By contrast, several studies demonstrated that early feeding with fluid diet or enteral nutrition through nasogastric tube after EVL had no adverse or favorable effect (11,12). More notably, a cohort study also demonstrated that early feeding with fluid diet could shorten the length of hospital stay (11). Generally, based on the current evidence, the liquid or soft diet might be preferred, while vigorous exercise and elevated abdominal pressure should be avoided to prevent from early slippage of ligation bands (13). We speculated that the early re-bleeding episode after EVL in our patient might be because the ligation bands slipped in advance after eating banana and then an unhealed ulcer was left. Certainly, we had to acknowledge that eating banana followed by re-bleeding might be a coincidence.
Several studies had reported the incidence and risk factors of early re-bleeding after EVL. Xu et al. reported the rate of early re-bleeding was 7.6% and most of re-bleeding episodes developed between the 7th and 13th days after EVL (14). Risk factors included volume of ascites, number of rubber bands used to ligate, severity of varices, and prolonged PT. Zhou et al. reported that the rate of early re-bleeding after EVL was 6.6% (15). Risk factors for early re-bleeding included male, a Child-Pugh score of >7.2, and volume of hematemesis. Lo et al. demonstrated that the early re-bleeding rate after EVL was approximately 10% (16). By comparison, Mostafa et al. reported a relatively high early re-bleeding rate after EVL (about 20%) (17). The most important risk factors of early re-bleeding after EVL were spontaneous bacterial peritonitis and low HB level. In general, the severity of liver dysfunction and complications in liver cirrhosis played a major role in prediction of early re-bleeding.
In such a patient, a second look by endoscopy should be actively considered (18). If necessary, a second endoscopic treatment should be given. However, this patient and his relatives refused a second endoscopy. Thus, we may not accurately determine the source of the bleeding. In this setting, a higher dose of vasoconstrictor can be attempted (3). Additionally, in patients who developed early re-bleeding after EVL, TIPS should be considered as a rescue therapy of choice (3,18). Balloon tamponade is only recommended for controlling massive bleeding as a temporary “bridge” (19). Esophageal stents can be considered for esophageal ulcer bleeding after EVL (18). Due to the restriction of medical resources, our patient received vasoconstrictors (i.e., terlipressin) and proton-pump inhibitor (i.e., esomeprazole) for the successful treatment of early re-bleeding after EVL.
In conclusion, based on the lessons from this case, bananas may not be given during the early postoperative period. The dietary principles after EVL need to be standardized to reduce the risk of early bleeding in future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823-32. [Crossref] [PubMed]
- Li Y, Han B, Li H, et al. Effect of Admission Time on the Outcomes of Liver Cirrhosis with Acute Upper Gastrointestinal Bleeding: Regular Hours versus Off-Hours Admission. Can J Gastroenterol Hepatol 2018;2018:3541365. [Crossref] [PubMed]
- de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743-52. [Crossref] [PubMed]
- Bai Z, Guo X, Li H, et al. Should red blood cell transfusion be immediately given to a cirrhotic patient with active upper gastrointestinal bleeding? AME Med J 2018;3:83. [Crossref]
- Zhou X, Tripathi D, Song T, et al. Terlipressin for the treatment of acute variceal bleeding: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97:e13437. [Crossref] [PubMed]
- Tripathi D, Stanley AJ, Hayes PC, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680-704. [Crossref] [PubMed]
- Aggeletopoulou I, Konstantakis C, Manolakopoulos S, et al. Role of band ligation for secondary prophylaxis of variceal bleeding. World J Gastroenterol 2018;24:2902-14. [Crossref] [PubMed]
- Lo GH. Endoscopic treatments for portal hypertension. Hepatol Int 2018;12:91-101. [Crossref] [PubMed]
- Cardenas A, Fernandez-Simon A, Escorcell A. Endoscopic band ligation and esophageal stents for acute variceal bleeding. Clin Liver Dis 2014;18:793-808. [Crossref] [PubMed]
- Hebuterne X, Vanbiervliet G. Feeding the patients with upper gastrointestinal bleeding. Curr Opin Clin Nutr Metab Care 2011;14:197-201. [Crossref] [PubMed]
- Lo GH, Lin CW, Hsu YC. A controlled trial of early versus delayed feeding following ligation in the control of acute esophageal variceal bleeding. J Chin Med Assoc 2015;78:642-7. [Crossref] [PubMed]
- de Ledinghen V, Beau P, Mannant PR, et al. Early feeding or enteral nutrition in patients with cirrhosis after bleeding from esophageal varices? A randomized controlled study. Dig Dis Sci 1997;42:536-41. [Crossref] [PubMed]
- Vanbiervliet G, Giudicelli-Bornard S, Piche T, et al. Predictive factors of bleeding related to post-banding ulcer following endoscopic variceal ligation in cirrhotic patients: a case-control study. Alimentary Pharmacology & Therapeutics 2010;32:225-32. [Crossref] [PubMed]
- Xu L, Ji F, Xu QW, et al. Risk factors for predicting early variceal rebleeding after endoscopic variceal ligation. World J Gastroenterol 2011;17:3347-52. [Crossref] [PubMed]
- Zhou JN, Wei Z, Sun ZQ. Risk factors for early rebleeding after esophageal variceal ligation in patients with liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi 2016;24:486-492. [PubMed]
- Lo GH, Chen WC, Chen MH, et al. Endoscopic ligation vs. nadolol in the prevention of first variceal bleeding in patients with cirrhosis. Gastrointest Endosc 2004;59:333-8. [Crossref] [PubMed]
- Mostafa EF, Mohammad AN. Incidence and predictors of rebleeding after band ligation of oesophageal varices. Arab Journal of Gastroenterology 2014;15:135-41. [Crossref] [PubMed]
- Hernandez-Gea V, Berbel C, Baiges A, et al. Acute variceal bleeding: risk stratification and management (including TIPS). Hepatol Int 2018;12:81-90. [Crossref] [PubMed]
- D'Amico M, Berzigotti A, Garcia-Pagan JC. Refractory acute variceal bleeding: what to do next? Clin Liver Dis 2010;14:297-305. [Crossref] [PubMed]
Cite this article as: Li Y, Guo X, Bai Z, Shao X, Wang R, Li H, Qi X. Banana may be forbidden after endoscopic variceal ligation: a case report. Transl Gastroenterol Hepatol 2019;4:13.


