
Endoscopic ultrasound guided biliary drainage: a comprehensive review
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is considered the first line intervention for pancreaticobiliary drainage for management of bile duct stones, benign and malignant biliary strictures (1-3), acute and chronic pancreatitis (4), pancreatic duct disruption (2), and numerous other disease processes. In cases when conventional ERCP is unsuccessful, it is recommended that a repeat ERCP should be performed by a more experienced endoscopist at a high-volume center (5). Although the expected success rate for ERCP is greater than 90%, there are factors preventing successful ERCP even by a skilled therapeutic endoscopist including surgically altered anatomy (i.e., Whipple, Roux-en-Y gastric bypass, Billroth II surgery), periampullary diverticula, gastric outlet obstruction, and malignant obstruction of the lumen (6-8). It was previously estimated that in the United States, 700,000 ERCPs are performed annually with a failure rate between 4–10% despite advanced techniques such as wire-guided cannulation, double wire technique or precut sphincterotomy (9,10).
Alternative options for biliary drainage after failure of conventional ERCP include percutaneous transhepatic biliary drainage (PTBD) and surgical biliary bypass (11,12). Although PTBD is a safer and more widely utilized alternative than surgery (13), complication rates have been estimated as high as 23%; this is most commonly due to cholangitis, dislocation or obstruction of catheters, higher likelihood for repeated biliary drainages, and patient dissatisfaction with indwelling catheter appearance and management (14-17).
Over the past several decades, endoscopic ultrasound (EUS) has transformed from strictly a diagnostic tool to a therapeutic technique allowing for the emergence of EUS-guided biliary drainage (EUS-BD) (18-21). This technique involves accessing the biliary tree with a fine-needle aspiration needle and guidewire, creating a fistulous tract with cautery and/or dilation, and ultimately deploying a decompressing stent under endosonographic and fluoroscopic visualization. In the past few years, there have been multiple retrospective and prospective studies evaluating the optimal techniques and indications for biliary drainage. We will review the most up-to-date indications and techniques in this review.
Indications
EUS-BD is indicated for benign and malignant biliary obstruction when conventional ERCP is unsuccessful or not feasible. The addition of endosonography allows for improved visualization of extraluminal structures as well as strategic technical planning for biliary drainage (22). The most common malignancies that require EUS-BD include pancreatic cancer, cholangiocarcinoma, and metastatic disease (23). Several small studies have investigated the use of EUS-BD as a first line approach for biliary decompression, instead of conventional ERCP, in the case of malignant biliary obstruction (24,25). Paik et al. (24) demonstrated in the first noninferiority trial that EUS-BD has comparable clinical and technical success to conventional ERCP with transpapillary stenting for primary palliation of malignant distal biliary obstruction. Furthermore, longer stent patency, less adverse rates and complications, and improved quality of life were associated with EUS-BD. However, larger randomized prospective trials are needed to adequately assess the role of EUS-BD in primary management of malignant obstruction.
Although one of the most common reasons for need of EUS-BD is obstructive jaundice due to underlying malignancy, it is crucial prior to any endoscopic intervention to assess if the patient is a candidate for curative surgical resection. If there is intent to proceed with surgical resection, then pre-operative biliary drainage via ERCP has been shown to increase rates of complications during surgery, with post-ERCP cholangitis as a predominant etiology (26). However in the case of a patient with underlying malignancy initially presenting with cholangitic symptoms or intense pruritus, then endoscopic biliary drainage can benefit the patient (27). This also applies in the case of patients who will require possible delay in surgical resection due to poor nutritional status or need for neoadjuvant chemotherapy. If ERCP or EUS-BD is pursued, transpapillary route is the preferred method. Complications from endoscopic biliary drainage, including cholangitis, hemorrhage, or pancreatitis could ultimately delay surgical resection and further therapy.
Technique
There are four well-described techniques for performing EUS-BD. The choice of the technique depends on the inherent reason for failed conventional ERCP, namely anatomic constraints, as well as the indication for biliary drainage and operator preference (22). The different techniques involve (I) the location of initial biliary access: intra-hepatic versus extra-hepatic; and (II) the location of stent deployment: transpapillary versus transgastric/transenteric. Intrahepatic approaches consist of accessing the biliary tree from the stomach through a dilated left intrahepatic duct (LIHD) and deploying a stent from the LIHD into the stomach [EUS-guided hepaticogastrostomy (EUS-HG)] or deploying a stent in a transpapillary antegrade fashion across a stricture or obstruction (EUS-antegrade stent placement). Extrahepatic approaches consist of accessing the biliary tree from the duodenal bulb through the common bile duct (CBD) or common hepatic duct (CHD) and deploying a stent from the CBD/CHD into the small bowel lumen [EUS-guided choledochoduodenostomy (EUS-CD)]. Stents can also be deployed in a transpapillary position using a rendez-vous technique from either an intra-hepatic or extra-hepatic approach (EUS-RV).
EUS-HG
EUS-HG is most commonly performed in cases of gastric outlet obstruction and post-surgical anatomy. Dilation of the left intrahepatic ducts, typically within segment three, is ideal for this approach (22). Absolute contraindications to HG include tumor infiltration along the stomach wall (due to risk for stent migration and bleeding); relative contraindications include massive ascites, coagulopathy, and lack of LIHD dilation (22). Stricture formation at the hepatic hilum may prevent successful decompression after EUS-HG due to inability to adequately drain the right intrahepatic system (28). However, there have been published cases of successful EUS-HG for right intrahepatic biliary drainage as well (29,30). Because this approach adds technical complexity, its use should be carefully determined.
To initiate EUS-HG placement, ultrasound is utilized to visualize the left intrahepatic bile ducts. Color doppler is utilized to visualize and avoid intervening vasculature. Transmural puncture from the proximal gastric body into the left intrahepatic bile duct is made using a 19-gauge needle (31,32) (Figure 1). Optimal transmural distance from the LIHD to the stomach wall estimated by EUS has been suggested to be between 1–3 cm with a goal bile duct diameter >5 mm (33). Once needle access has been obtained, contrast is injected to obtain a cholangiogram to delineate biliary anatomy. A guidewire is then advanced through the needle into the intrahepatic ducts. A 450 cm wire is typically used, though shorter 240 cm guidewire has been used in some studies to allow for faster manipulation of endoscopic accessory tools (34). Once the guidewire is advanced into the bile duct towards the hilum, fistulous tract creation is performed using a dilating balloon. In some cases, cautery is required to create the fistulous tract prior to dilation. Finally, stent deployment over the guidewire is completed to achieve hepatico-gastric biliary drainage. In most cases, metal stents are preferred to best prevent bile leakage.
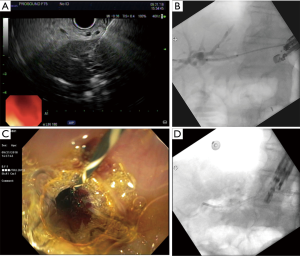
EUS-antegrade stent placement
The technique for an EUS-antegrade approach is similar to that used in EUS-HG. The notable difference is that the stent is deployed across the area of obstruction within the bile duct. It is crucial in stent deployment that the guidewire actually traverses the area of obstruction within the biliary tree (22). As described above, transmural puncture of the LIHD with 19-gauge needle is performed from the stomach and contrast is injected to obtain a cholangiogram. A guidewire is advanced into the left intrahepatic ducts and directed across the area of biliary obstruction into the small intestine. Repeat contrast injection is then recommended to confirm filling of the small intestine and localize the biliary-enteric junction. Stent deployment is then performed in an antegrade fashion, advancing the stent through the LIHDs then across the stricture and preferably across the ampulla (35,36) (Figure 2). Metal stents are typically preferred, though plastic stents can also be utilized. After stent deployment, the gastric puncture site can be closed with a hemoclip or a HG stent can be deployed for continued biliary access (22).
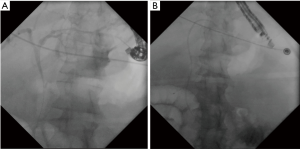
EUS-CD
EUS-CD is a reasonable approach when papillary access is not feasible and the extrahepatic portion of the CBD is dilated due to distal obstruction. In similar fashion to EUS-HG, needle puncture with 19-gauge needle is performed under ultrasound guidance, a cholangiogram is obtained, and a guidewire is inserted from the duodenum towards the main biliary confluence (37) (Figure 3). Fistulous tract creation with balloon dilation and/or cautery is then performed. A stent is then deployed between the duodenal lumen and the extra-hepatic biliary tree. Metal stents are most commonly utilized with plastic stents for anchoring. However, lumen-apposing metal stents (LAMS) are being shown to have high technical and clinical success and are in the process of being developed specifically for EUS-CD (38).
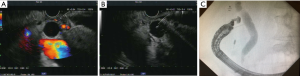
EUS-RV
In circumstances when the second portion of the duodenum is accessible yet conventional ERCP (in the retrograde position) via the papilla is not feasible, EUS-RV can be considered for biliary access and drainage. Particular scenario when this might arise include presence of a large periampullary diverticulum or ampullary tumor (39). This technique was first highlighted in a few small case series starting in 2004 (40,41). It has also been suggested to be superior to precut papillotomy for procedural success, although there is no difference in procedural complications (10,42). In this technique, biliary access is obtained via techniques as outlined above and the guidewire is directed across the ampulla deep into the small bowel. However, instead of fistula creation with balloon dilation and/or cautery, the guidewire is left in place while the echoendoscope is removed using an exchange technique. A standard duodenoscope is then inserted to the level of the papilla, the wire is grasped using forceps or a snare and withdrawn through the accessory channel in the scope, and a conventional ERCP procedure is performed (Figure 4).
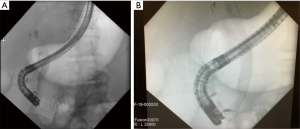
Choosing your technical approach
Currently, there is no consensus on the ideal route to approach biliary drainage. Thus, it is often at the discretion and judgement of the advanced endoscopist (43). Given the myriad of approaches to endoscopic drainage for the same level of biliary obstruction, the importance of identifying the safest and most effective route of drainage is paramount to clinical success.
To date there have been a few comparative studies for safety and efficacy of the different endoscopic approaches with varied results, with majority of data arising from retrospective reviews. In a study by Artifon et al., technical success rate was 91% in a census of 24 patients that underwent EUS-CDS, comparable to a success rate of 96% for the EUS-HG approach (31). There was no statistical difference between the two techniques. In a comparative study of EUS-transpapillary stenting versus EUS-CD, shorter procedural times and elimination of the pancreatitis risk made EUS-CD a worthwhile contender for first line palliative therapy in distal biliary obstruction (44). In a multicenter, nonrandomized, retrospective analysis by Gupta et al. (45), no significant difference was noted in clinical success between intrahepatic and extrahepatic approaches. These results were verified in a prospective randomized trial in a tertiary care endoscopy center with 49 patients; no difference in outcome was noted between EUS-HG vs. EUS-CD (31). A large meta-analysis by Uemura et al. showed that EUS-HG and EUS-CD have equal efficacy and safety (23).
When EUS-RV is attempted, there has been suggestion that the extrahepatic route has improved safety compared to intrahepatic approach. Dhir et al. (46) demonstrated in a retrospective analysis of 35 patients that increased hospitalization time, longer procedure time, and increased post-procedure pain are associated with the transhepatic route for the rendez-vous approach. In another retrospective study two years later by Dhir et al. comparing 68 patients among all four ultrasound-guided procedures, the success rate was high among all approaches regardless of access, stent direction or drainage route (47). However, increased complications were seen in transhepatic route versus the transduodenal approach (bleeding, cholangitis, perforation, bile leak, pneumoperitoneum, death) with a significant P value. On the contrary, in a large systematic analysis of 1,192 patients by Wang et al., the authors demonstrated no difference between the technical success rate, functional success rate, or adverse event rate of transgastric and transduodenal biliary approaches (19). In cases of obstructive jaundice with concomitant duodenal obstruction with need for both biliary and duodenal decompression with stenting, EUS-HG may be the preferred route due to longer stent patency and fewer adverse events (48).
An algorithm to standardize management of biliary obstruction was proposed in 2016 (49). After cross-sectional imaging is attained, demonstration of intra- or extra-hepatic dilation can determine preferred route of biliary drainage. If the intrahepatic biliary tree is dilated, it is recommended that anterograde approach via the intrahepatics be attempted first. If unsuccessful, procedure can be converted to EUS-HG. If HG is unsuccessful, then at this point EUS-rendez-vous technique should be considered, and if this is not feasible, then ultimately a transenteric stent should be placed. If the intrahepatic tree is not dilated, then the decision proposed is: first, rendez-vous technique should be attempted, and if it cannot be achieved successfully, then transenteric stent placement should be performed. This algorithm was suggested on the principle that endoscopic technique should be decided based on the preferred preservation of natural hepatobiliary anatomy. In application of this algorithm for 52 patients, no difference in safety or efficacy was noted between intrahepatic or extrahepatic approaches.
Safety and efficacy of EUS-BD
Success of EUS-guided biliary drainage procedures is remarkably high, with pooled data showing up to 94% technical success rate and 90% clinical success rate (19,24). Advantages of EUS-biliary drainage include ease of access to bile ducts when the ampulla is not easily accessible endoscopically, and avoidance of papillary interventions that could lead to acute pancreatitis (24). When conventional ERCP fails, it is possible to convert to EUS biliary drainage within the same procedure, avoiding the need for repeat procedures and delayed ductal decompression. Furthermore, EUS-BD can allow for longer stent patency (50). This naturally leads to decreased patient costs for repeated procedures and improves patient satisfaction.
Compared to surgical intervention, i.e., hepaticojejunostomy, for malignant biliary obstruction, a small randomized study by Artifon et al. showed that EUS-BD (in the EUS-CD approach) had shorter procedural times with no difference in adverse events or quality of life scores (51). Another advantage when compared to PTBD is that use of EUS allows visualization of vasculature using doppler imaging to prevent accidental vascular injury (52).
The risks associated with EUS-BD are varied, but the complication rates appear to be decreasing as experience with EUS-BD is growing in tertiary care centers (53). In a recent meta-analysis of 42 studies with 1,192 patients, an adverse event rate for EUS guided biliary drainage was calculated as 23%. The risk of bleeding and bile leakage were highest at 4.03% followed by lower risk of pneumoperitoneum, stent migration, cholangitis, abdominal pain and peritonitis (19). It is highly recommended that EUS-BD be performed in high volume tertiary care endoscopy centers, where there is endoscopic expertise along with support from surgical and interventional radiology departments.
Conclusions
Rapid endoscopic advances in biliary decompression have been made over the past 10 years. With the emergence of the field of therapeutic EUS, there are new and innovative ways to safely drain the hepatobiliary system in patients who cannot undergo conventional ERCP. As a result, the breadth and depth of endoscopic offerings to patients has drastically increased and invasive procedures can be avoided. With four described endoscopic techniques—EUS-HG, EUS-CD, EUS-RV, and EUS-antegrade stent placement—there are a multitude of safe and effective approaches for biliary decompression. Optimal approaches to EUS-guided biliary drainage for hepatobiliary decompression continue to be investigated and improved, allowing for the emergence of a cohesive approach for patient care.
Acknowledgements
None.
Footnote
Conflicts of Interest: Amy Tyberg has done consultant work for EndoGastric Solutions, NinePoint Medical, and Obalon Therapeutics Inc. Avantika Mishra has no conflicts of interest to declare.
References
- Fogel EL, Sherman S, Devereaux B, et al. Therapeutic biliary endoscopy. Endoscopy 2003;35:156-63. [Crossref] [PubMed]
- Carr-Locke DL. Overview of the role of ERCP in the management of diseases of the biliary tract and the pancreas. Gastrointest Endosc 2002;56:S157-60. [Crossref] [PubMed]
- Schöfl R. Diagnostic endoscopic retrograde cholangiopancreatography. Endoscopy 2001;33:147-57. [Crossref] [PubMed]
- Sarkaria S, Lee HS, Gaidhane M, et al. Advances in endoscopic ultrasound-guided biliary drainage: a comprehensive review. Gut Liver 2013;7:129-36. [Crossref] [PubMed]
- Kumar S, Sherman S, Hawes RH, et al. Success and yield of second attempt ERCP. Gastrointest Endosc 1995;41:445-7. [Crossref] [PubMed]
- Baron TH, Petersen BT, Mergener K, et al. Quality Indicators for Endoscopic Retrograde Cholangiopancreatography. Am J Gastroenterol 2006;101:892. [Crossref] [PubMed]
- Enochsson L, Swahn F, Arnelo F, et al. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest Endosc 2010;72:1175-84, 1184.e1-3.
- Minaga K, Kitano M. Recent advances in endoscopic ultrasound-guided biliary drainage. Dig Endosc 2018;30:38-47. [Crossref] [PubMed]
- Coté GA, Singh S, Bucksot LG, et al. Association between volume of endoscopic retrograde cholangiopancreatography at an academic medical center and use of pancreatobiliary therapy. Clin Gastroenterol Hepatol 2012;10:920-4. [Crossref] [PubMed]
- Itoi T, Dhir V. EUS-guided biliary rendezvous: slow, hesitant, baby steps forward. Gastrointest Endosc 2016;83:401-3. [Crossref] [PubMed]
- Pollock TW, Ring ER, Oleaga JA, et al. Percutaneous decompression of benign and malignant biliary obstruction. Arch Surg 1979;114:148-51. [Crossref] [PubMed]
- Ferrucci JT Jr, Mueller PR, Harbin WP. Percutaneous transhepatic biliary drainage: technique, results, and applications. Radiology 1980;135:1-13. [Crossref] [PubMed]
- Holt BA, Hawes R, Hasan M, et al. Biliary drainage: role of EUS guidance. Gastrointest Endosc 2016;83:160-5. [Crossref] [PubMed]
- Voegeli DR, Crummy AB, Weese JL. Percutaneous transhepatic cholangiography drainage, and biopsy in patients with malignant biliary obstruction: An alternative to surgery. Am J Surg 1985;150:243-7. [Crossref] [PubMed]
- Oh HC, Lee SK, Lee TY, et al. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy 2007;39:731-6. [Crossref] [PubMed]
- Sharaiha RZ, Kumta NA, Desai AP, et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg Endosc 2016;30:5500-5. [Crossref] [PubMed]
- Nennstiel S, Weber A, Frick G, et al. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis Over 10 Years. J Clin Gastroenterol 2015;49:764-70. [Crossref] [PubMed]
- Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy 2001;33:898-900. [Crossref] [PubMed]
- Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: a systematic review. Gastrointest Endosc 2016;83:1218-27. [Crossref] [PubMed]
- Kahaleh M, Hernandez AJ, Tokar J, et al. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc 2006;64:52-9. [Crossref] [PubMed]
- Lee TH, Choi JH. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin Gastroenterol Hepatol 2016;14:1011-9.e3. [Crossref] [PubMed]
- Boulay BR, Lo SK. Endoscopic Ultrasound-Guided Biliary Drainage. Gastrointest Endosc Clin N Am 2018;28:171-85. [Crossref] [PubMed]
- Uemura RS, Khan MA, Otoch JP, et al. EUS-guided Choledochoduodenostomy Versus Hepaticogastrostomy: A Systematic Review and Meta-analysis. J Clin Gastroenterol 2018;52:123-30. [PubMed]
- Paik WH, Lee TH, Park DH, et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol 2018;113:987-97. [Crossref] [PubMed]
- Bang JY, Navaneethan U, Hasan M, et al. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc 2018;88:9-17. [Crossref] [PubMed]
- van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 2010;362:129-37. [Crossref] [PubMed]
- Domínguez-Muñoz JE, Lariño-Noia J, Iglesias-Garcia J. Biliary drainage in pancreatic cancer: The endoscopic retrograde cholangiopancreatography perspective. Endoscopic Ultrasound 2017;6:S119-21. [Crossref] [PubMed]
- Ogura T, Higuchi K. Technical tips for endoscopic ultrasound-guided hepaticogastrostomy. World J Gastroenterol 2016;22:3945-51. [Crossref] [PubMed]
- Ogura T, Sano T, Onda S, et al. Endoscopic ultrasound-guided biliary drainage for right hepatic bile duct obstruction: novel technical tips. Endoscopy 2015;47:72-5. [PubMed]
- Park SJ, Choi JH, Park DH, et al. Expanding indication: EUS-guided hepaticoduodenostomy for isolated right intrahepatic duct obstruction (with video). Gastrointest Endosc 2013;78:374-80. [Crossref] [PubMed]
- Artifon EL, Marson FP, Gaidhane M, et al. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: is there any difference? Gastrointest Endosc 2015;81:950-9. [Crossref] [PubMed]
- Yamao K, Hara K, Mizuno N, et al. EUS-Guided Biliary Drainage. Gut Liver 2010;4:S67-75. [Crossref] [PubMed]
- Oh D, Park DJ, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol 2017;10:42-53. [Crossref] [PubMed]
- Teoh AYB, Dhir V, Kida M, et al. Consensus guidelines on the optimal management in interventional EUS procedures: results from the Asian EUS group RAND/UCLA expert panel. Gut 2018;67:1209-28. [Crossref] [PubMed]
- Imai H, Takenaka M, Omoto S, et al. Utility of Endoscopic Ultrasound-Guided Hepaticogastrostomy with Antegrade Stenting for Malignant Biliary Obstruction after Failed Endoscopic Retrograde Cholangiopancreatography. Oncology 2017;93:69-75. [Crossref] [PubMed]
- Godat S, Bories E, Caillol F, et al. Efficacy and safety in case of technical success of endoscopic ultrasound-guided transhepatic antegrade biliary drainage: A report of a monocentric study. Endosc Ultrasound 2017;6:181-6. [Crossref] [PubMed]
- Kumbhari V, Tieu AH, Khashab MA. EUS-guided biliary drainage made safer by a combination of hepaticogastrostomy and antegrade transpapillary stenting. Gastrointest Endosc 2015;81:1015-6. [Crossref] [PubMed]
- Tsuchiya T, Teoh AYB, Itoi T, et al. Long-term outcomes of EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction: a prospective multicenter study. Gastrointest Endosc 2018;87:1138-46. [Crossref] [PubMed]
- Mangiavillano B, Arcidiacono PG, Carrara S, et al. EUS-guided rendezvous technique for difficult cannulation of an intradiverticular papilla. Endoscopy 2008;40:E87-8. [Crossref] [PubMed]
- Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: report of 6 cases. Gastrointest Endosc 2004;59:100-7. [Crossref] [PubMed]
- Kahaleh M, Wang P, Shami VM, et al. EUS-guided transhepatic cholangiography: report of 6 cases. Gastrointest Endosc 2005;61:307-13. [Crossref] [PubMed]
- Dhir V, Bhandari S, Bapat M, et al. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointest Endosc 2012;75:354-9. [Crossref] [PubMed]
- Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc 2017;85:904-14. [Crossref] [PubMed]
- Kawakubo K, Kawakami H, Kuwatani M, et al. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy 2016;48:164-9. [PubMed]
- Gupta K, Perez-Miranda M, Kahaleh M, et al. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol 2014;48:80-7. [Crossref] [PubMed]
- Dhir V, Bhandari S, Bapat M, et al. Comparison of transhepatic and extrahepatic routes for EUS-guided rendezvous procedure for distal CBD obstruction. United European Gastroenterol J 2013;1:103-8. [Crossref] [PubMed]
- Dhir V, Artifon EL, Gupta K, et al. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: choice of access route, direction of stent insertion, and drainage route. Dig Endosc 2014;26:430-5. [Crossref] [PubMed]
- Ogura T, Chiba Y, Masuda D, et al. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy 2016;48:156-63. [PubMed]
- Tyberg A, Desai AP, Kumta NA, et al. EUS-guided biliary drainage after failed ERCP: a novel algorithm individualized based on patient anatomy. Gastrointest Endosc 2016;84:941-6. [Crossref] [PubMed]
- Khashab MA, Valeshabad AK, Afghani E, et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci 2015;60:557-65. [Crossref] [PubMed]
- Artifon EL, Loureiro JF, Baron TH, et al. Surgery or EUS-guided choledochoduodenostomy for malignant distal biliary obstruction after ERCP failure. Endosc Ultrasound 2015;4:235-43. [Crossref] [PubMed]
- Shami VM, Kahaleh M. Endoscopic ultrasound-guided cholangiopancreatography and rendezvous techniques. Dig Liver Dis 2010;42:419-24. [Crossref] [PubMed]
- Khashab MA, Van der Merwe S, Kunda R, et al. Prospective international multicenter study on endoscopic ultrasound-guided biliary drainage for patients with malignant distal biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Endosc Int Open 2016;4:E487-96. [Crossref] [PubMed]
Cite this article as: Mishra A, Tyberg A. Endoscopic ultrasound guided biliary drainage: a comprehensive review. Transl Gastroenterol Hepatol 2019;4:10.


