Palliative therapy in pancreatic cancer—interventional treatment with stents
Introduction
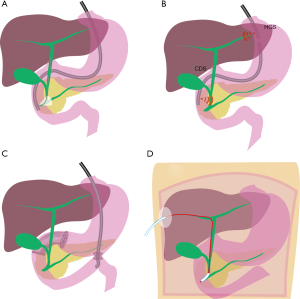
Interventional treatment with stents in a palliative setting is a topic that developed on a large scale since its first description by Nib Soehendra in 1979 and its development seems to even accelerate in the more recent past (1). Besides the most obvious and best established use of biliary stents in endoscopic retrograde cholangiopancreatography (ERCP) for palliative endoscopic treatment of obstructive jaundice in pancreatic cancer (2), several other fields of palliative stent therapy have evolved (Figure 1), that will be addressed in detail here.
Methods
A PubMed and Google Scholar search with the terms “pancreatic cancer” and “stent” was performed and evaluated from 2018 reversely to 2010 to identify suitable studies. 776 studies where identified on PubMed and 74 on Google Scholar (title). Further specification of the search by adding terms EUS or ERCP reduced the results to 110/3 as well as 241/4 respectively (PubMed/Google Scholar). The results were evaluated by abstract content for eligibility. When the content was suitable, a lecture of the full publication with evaluation of the specific references was performed. Additional hand search within references revealed further information on publications and details possibly of interest for this review. Furthermore guidelines of the ASGE (3) and ESGE (2) were considered and the evidence for current practice was reviewed.
Retrograde transpapillary biliary stenting via ERCP
Painless obstructive jaundice is a hallmark in pancreatic cancer and is most commonly caused by a compression and obstruction of the intrapancreatic portion of the ductus choledochus by the tumor. In most cases this situation can be addressed by implantation of plastic- or self-expanding metal stents (SEMS) in the biliary tract via ERCP (Figure 1A) (4). The choice of stent in this setting has extensively been discussed from several viewpoints. Plastic stents are less expensive than SEMS, but it is known that in the biliary tract they occlude because of bacterial colonization, with the most cited time span of 3 to 6 months until stent occlusion, increasing the risk for recurrent jaundice and cholangitis (5). SEMS exist in covered and uncovered versions and both have significantly longer patency rates than plastic stents that exceed the life expectancy of most patients with metastatic pancreatic cancer (6,7). A variety of studies showed quite homogeneously a similar stent patency in both SEMS types, with better control of tumor ingrowth, but higher rate of tumor overgrowth as well as more problems with stent migration in the covered SEMS group (6-9). Specific weaknesses of stent types have partially addressed: covered SEMS have been developed towards preventing dislocation. In uncovered SEMS the implantation of an additional covered SEMS into an indwelled uncovered SEMS can enable successful explantation of both stents in the further course (10). Partially covered SEMS are developed for uniting the best of both covered and uncovered SEMS, but it is difficult to give general statements on this heterogeneous group of stents. An issue that has been repeatedly brought up is the risk of cholecystitis due to implantation of a covered SEMS (6,11,12). Meta-analyses on this topic for the most do not find convincing evidence of higher rates of cholecystitis in covered compared to uncovered SEMS (7,9,13,14) and publications looking more detailed into patients characteristics identified tumor invasion of the feeding artery of the gallbladder and the orifice of the cystic duct as risk factors for cholecystitis after SEMS placement (15,16). Newer guidelines suggest the implantation of SEMS for all palliative cases (2,3), while earlier suggestions referred to thresholds of patients estimated life expectancy between 2 and 6 months for cost effectiveness of SEMS implantation (17,18). The cost of an ERCP with stent implantation of course varies in different parts of the world, depending on expenses for medical care as well as material, including taxes and shipping costs. In highly developed countries, stent costs do not represent the majority of the expenses in the palliative care of patients with malignant extrahepatic biliary obstructions, independently of the stent choice. In a Dutch multicenter study, the cost difference for the initial ERCP was purely depending on the price of the stent used (plastic 1,106$ vs. SEMS 2,094$), while the total costs of care did not differ after a follow up of up to 1 year (plastic stents $7,770 and SEMS $7,356). And this applied even in a patient subgroup who survived less than 3 months (19). In an American study cost was lower in patients treated with SEMS than with plastic stents due to fewer stent exchange procedures (18). Even in countries with relatively low ERCP costs in relation to the stent prices, the mean total cost of the relief of jaundice is not significantly different between SEMS (1,488.77$) and plastic stent patients (1,319.26$) due to less frequent and shorter hospitalization for cholangitis in the SEMS group in patients with unresectable malignant biliary obstruction (20).
The use of SEMS is encouraged by ESGE also for bilateral intrahepatic strictures, either in side by side- or SEMS through SEMS-technique. Uncovered SEMS should be used to avoid possible complications caused by duct occlusion like incomplete drainage, cholangitis and abscess formation by (2). Plastic stenting for intrahepatic strictures is recommended if there is reasonable doubt on the malignant nature of the stricture, as well as in the case of SEMS obstruction by tumor ingrowth. In the last case the additional placement of either a second SEMS in SEMS as well as a plastic stent in the occluded SEMS are possible endoscopic options (2).
In patient with malignant hilar or intrahepatic stenosis, ERCP should aim at draining at least 50% of the liver volume and only to opacity ducts that will be drained during the intervention to avoid complications (21,22).
We interpret the ESGE’s recent recommendation for almost exclusive SEMS implantation in pancreatic cancer as a consequence of increasing evidence that plastic stent patency in patients with pancreatic cancer is often shorter than the frequently citied 3 to 6 months (23) together with a less predictable life expectancy under modern treatment regimens. More seems to be at stake in the likely event of stent dysfunction, especially under aggressive chemotherapy, possibly resulting in a wide field of undesired effects ranging from additional diagnostic imaging and therapeutic drainage procedures with all related costs and patients stress, over delayed or discontinued chemotherapy, severe cholangitis, organ dysfunction to significant reduction of survival and even death (24). Still, this recommendation has not yet been fully applied in endoscopy units in our field of view. This might be due to the multiplying material costs of several SEMS as well as less experience in the use of SEMS in cases with widespread or metastatic disease and complex compression of intrahepatic ducts.
Percutaneous transhepatic cholangiography (PTC)
PTC (Figure 1D) has been challenged by endoscopic and lately EUS approaches and the frequency of PTC has gradually decreased (25,26). The insertion of a wire for rendezvous, a plastic drain for internal and/or external (Yamakawa-/Münchner-drainage) as well as a metal stent are well established, but there is a higher risk for complications and impact on quality of life compared to EUS approaches (27). Although these percutaneous interventions represent an appreciated salvage procedure, if local EUS expertise is available, PTC should be considered only after both ERCP and EUS-BD attempts have failed or are not suitable (27).
Endosonographic biliary drainage (EUS-BD)
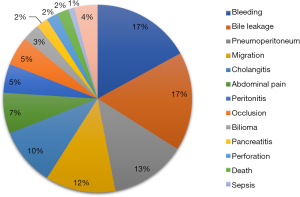
Cannulation of the papilla Vateri is successful in a vast majority of ERCP attempts (4), but in some cases an alternative access for biliary drainage and stent implantation has to be established. Giovannini published the first report on transduodenal endosonography (EUS) guided rendezvous with stent implantation after a failed ERCP attempt in 2001 (28). The first direct biliary drainage with stent implantation was reported in 2003 by Burmester, who used plastic stents (29) and shortly later by Giovannini using a SEMS (30). Since then several endosonographic access routes to the biliary tract have been described with minor variations in mostly small patient groups that can mainly be categorized into intrahepatic and extrahepatic access (Figure 1B). Stent placement can be performed bilioenteric [i.e., hepaticogastrostomy (HGS), cholecystogastrostomy or choledochoenterostomy (CDS)] or transpapillary, either antegrade or via rendezvous procedure (31). In the largest meta-analysis on EUS-BD so far, higher complications rates for EUS-BD compared to ERCP have been described (32), but chances for complications might be higher in selected cases with failed primary biliary cannulation. A retrospective study found a higher complication rate for endoscopic precut papillotomy than for EUS guided rendezvous after failed primary biliary cannulation (33). Only recently three prospective randomized trails comparing ERCP and EUS-BD were published including 62.4%, 90% respective 100% patients with pancreatic cancer as a cause for malignant biliary obstruction (34-36). The study by Bang et al. reported no statistical differences in any of the outcome measures between the two groups (36) and Park et al. observed very similar results with absence of early complications and a difference in the of type of late adverse events: four cases of SEMS tumor overgrowth in the ERCP group, two cases of food impaction in the stent and two cases of late stent dislocation after a stable fistula had established in the EUS group (35). In the multicenter study by Paik et al. significantly less early and late complications as well as longer stent patency, fewer reinterventions, shorter procedure time and better quality of life in the follow up were reported while similar technical success rates were observed (34). Importantly, all cases that could not be addressed by the randomly assigned drainage procedure, both in the ERCP and the EUS group, crossed successfully over to the other group and there was no need to apply non endoscopic drainage techniques in all three studies. Details on the studies are listed in Table 1 and the types of complications observed in the meta-analysis by Wang et al. (32) are stated in Figure 2. These inconsistent results may at least partially be seen as a hen and egg problem: EUS BD is often performed as a rescue procedure, which applies to only 0.6–3.3% of the cases scheduled for ERCP in retrospective studies in tertiary care centers (37,38), while in the study centers of the three prospective studies comparing ERCP to EUS, the endoscopist’s expertise most likely exceeds the one found in an average tertiary care center. A single endoscopist experienced in both EUS and ERCP required an experience of 33 cases in EUS-BD to achieve a flattening of the learning curve with reduction of complications and procedure time were seen (39). Similar results were reported in a single center evaluation of the first 101 cases of EUS BD over 7 years: in the first half of the patients 5 procedure related death were observed, while in the second half only a single lethal complication occurred (40). In both measures, it seems to take several years of intense practice in an average tertiary center to achieve expertise to reduce interventional complications. Meta-analyses comparing extrahepatic and intrahepatic access routes for EUS-BD fund similar technical and functional success rates in both routes but adverse events were less frequent with the extrahepatic route in one analysis (32,41,42). In essence the choice of access route is dependent on the factors of patient’s anatomy, local expertise and personal preference (42,43). When it comes to choice of stents for EUS-BD the aim is to establish a tight connection between the gastrointestinal and the biliary tract to avoid biliary leakage and peritonitis. Expandable stents with a cover fit this aim better than plastic stents. Furthermore, the compression and expansion effect of the SEMS might be more useful to prevent the other common adverse events such as bleeding, stent obstruction and dislocation more than plastic stents (32,44). Among SEMS in EUS BD, an uncovered part is important for intrahepatic placement to avoid branch duct obstruction, while a longer covered part is useful in addressing the risk of stent dislocation and consecutive leakage, which seems to be highest in EUS HGS, since there can be significantly movement between the stomach and the liver (45). For this reason, a long unilaterally covered stent with an uncovered intrahepatic end is the favored design today in EUS HGS (45,46), while in the other access routes special SEMS are being developed and tested without a clear prevailing special design modification. Second generation LAMS, although primarily intended for the management of fluid collections, are appreciated for EUS-BD for the advantage of a single step stent insertion without time consuming and possibly dangerous device changes over the wire as well as a possibly better prevention of dislocation by the stents tulips in extrahepatic access in comparison to normal SEMS, especially when it comes to punction of the gallbladder (31,47).
Pancreatic stents for treatment for pain
Pain of “obstructive type” caused by pancreatic duct obstruction is an established concept in chronic pancreatitis than can be treated by decompression by implantation of a pancreatic ductal stent (48,49). Similar, but less evidence exists for decompression of the pancreatic duct by pancreatic cancer concerning amelioration of pain, opioid consumption and quality of life (50-53). All publications state that the procedure is safe and no excessive complication rates have been reported. Although the pathophysiological concept is convincing, data seem promising and the equipment to perform this procedure is easily available, we could not find evidence of a widely accepted practice of this technique or recent publications on this topic. EUS drainage of the pancreatic duct has been described in case reports in benign conditions (54) as well as in unresectable pancreatic cancer (55) as a salvage access route in patients with strong indication for ductal drainage because of recurrent pancreatitis, but to our knowledge no study data exist on this topic.
Gastroduodenal obstruction
Duodenal or gastric outlet obstruction is common in patients with advanced pancreatic cancer and the most established therapeutic options for this problem are operative gastroenterostomy and the implantation of an endoluminal gastroduodenal SEMS (GDS) (Figure 1C). Retrospective comparative studies quite homogeneously showed a shorter in hospitalization without significant differences in complications, reinterventions and survival between the groups (42,56,57), with higher score of patency for the surgical patients in the early follow up, but similar rates in the later follow up and earlier oral food intake and shorter time to chemotherapy in the GDS groups (42,57). The complications and risks of GDS include stent migration and blockage from food, debris or tumor ingrowth, as well as bleeding, perforation and blocking the ampulla, possibly causing pancreatitis or cholangitis. With improvement in life expectancies, these complications are becoming more relevant and patients need to be informed about dietary limitations to avoid stent occlusion. Often patients require luminal as well as biliary stenting in the course of the disease. While implantation of a GDS in patients with earlier biliary stenting is usually not a problem, the implantation of a biliary stent after an GDS implantation can be technically challenging and has lower success rates than normal ERCP (58,59). Therefore careful evaluation of the necessity of stenting of the biliary tract should be done before implantation of a duodenal stent. When obstructive jaundice arises after GDS implantation, EUS-BD provides an effective solution in this setting with significantly higher technical and clinical success rates than a classic ERCP approach (58) and EUS-BD via HGS provides longer biliary stent patency than via CDS (60).
EUS guided gastroenterostomy (GE) is a rapidly developing field which has first been described in 2002 (61) in animals and in 2005 in humans with the help of magnets (62). The technical feasibility has evolved by introduction of second generation LAMS and technical variations described range from direct punction to balloon assisted punction with single or double balloon catheters inserted through the stricture (63). Recent multicenter studies on this topic revealed lower complication rates when compared to surgical GE with similar clinical success rates in both groups (64,65), making EUS GE a promising alternative to both gastroduodenal stenting and surgical GE.
In conclusion, the palliative management of pancreatic cancer with stents is a quickly developing field, especially in EUS and SEMS applications. The various access routes for stent therapy in pancreatic cancer are illustrated in Figure 1. In our opinion it is important for endoscopists who perform ERCP in tertiary care centers to have knowledge and skills in EUS interventions as well, since the two methods develop towards a complementary use depending on individual patient’s properties.
Acknowledgements
This is publication PAN 18-32 from the Karolinska Pancreas team.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Soehendra N, Reynders-Frederix V. Palliative biliary duct drainage. A new method for endoscopic introduction of a new drain. Dtsch Med Wochenschr 1979;104:206-7. [Crossref] [PubMed]
- Dumonceau JM, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy 2018;50:910-30. [Crossref] [PubMed]
- Eloubeidi MA, Decker GA, Chandrasekhara V, et al. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc 2016;83:17-28. [Crossref] [PubMed]
- Enochsson L, Swahn F, Arnelo U, et al. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest Endosc 2010;72:1175-84, 1184.
- Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc 2003;57:178-82. [Crossref] [PubMed]
- Yoon WJ, Lee JK, Lee KH, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc 2006;63:996-1000. [Crossref] [PubMed]
- Li J, Li T, Sun P, et al. Covered versus Uncovered Self-Expandable Metal Stents for Managing Malignant Distal Biliary Obstruction: A Meta-Analysis. PLoS One 2016;11:e0149066. [Crossref] [PubMed]
- Yang MJ, Kim JH, Yoo BM, et al. Partially covered versus uncovered self-expandable nitinol stents with anti-migration properties for the palliation of malignant distal biliary obstruction: A randomized controlled trial. Scand J Gastroenterol 2015;50:1490-9. [Crossref] [PubMed]
- Almadi MA, Barkun AN, Martel M. No benefit of covered vs uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: a meta-analysis. Clin Gastroenterol Hepatol 2013;11:27-37.e1. [Crossref] [PubMed]
- Gonzalez N, Ramesh J, Wilcox CM, et al. Endoscopic removal of an impacted bile duct self-expanding metal stent (SEMS) using the SEMS-in-SEMS technique. Endoscopy 2013;45 Suppl 2 UCTN:E254-E255.
- Costamagna G, Tringali A, Reddy DN, et al. A new partially covered nitinol stent for palliative treatment of malignant bile duct obstruction: a multicenter single-arm prospective study. Endoscopy 2011;43:317-24. [Crossref] [PubMed]
- Tsuchiya T, Itoi T, Gotoda T, et al. A multicenter prospective study of the short-term outcome of a newly developed partially covered self-expandable metallic biliary stent (WallFlex((R))) Dig Dis Sci 2011;56:1889-95. [Crossref] [PubMed]
- Jang S, Stevens T, Parsi M, et al. Association of covered metallic stents with cholecystitis and stent migration in malignant biliary stricture. Gastrointest Endosc 2018;87:1061-70. [Crossref] [PubMed]
- Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc 2011;74:321-7.e1. [Crossref] [PubMed]
- Sogabe Y, Kodama Y, Honjo H, et al. Tumor invasion to the arteries feeding the gallbladder as a novel risk factor for cholecystitis after metallic stent placement in distal malignant biliary obstruction. Dig Endosc 2018;30:380-7. [Crossref] [PubMed]
- Shimizu S, Naitoh I, Nakazawa T, et al. Predictive factors for pancreatitis and cholecystitis in endoscopic covered metal stenting for distal malignant biliary obstruction. J Gastroenterol Hepatol 2013;28:68-72. [Crossref] [PubMed]
- Daróczi T, Bor R, Fabian A, et al. Cost-effectiveness trial of self-expandable metal stents and plastic biliary stents in malignant biliary obstruction. Orv Hetil 2016;157:268-74. [PubMed]
- Martinez JM, Anene A, Bentley TG, et al. Cost Effectiveness of Metal Stents in Relieving Obstructive Jaundice in Patients with Pancreatic Cancer. J Gastrointest Cancer 2017;48:58-65. [Crossref] [PubMed]
- Walter D, van Boeckel PG, Groenen MJ, et al. Cost Efficacy of Metal Stents for Palliation of Extrahepatic Bile Duct Obstruction in a Randomized Controlled Trial. Gastroenterology 2015;149:130-8. [Crossref] [PubMed]
- Yoon WJ, Ryu JK, Yang KY, et al. A comparison of metal and plastic stents for the relief of jaundice in unresectable malignant biliary obstruction in Korea: an emphasis on cost-effectiveness in a country with a low ERCP cost. Gastrointest Endosc 2009;70:284-9. [Crossref] [PubMed]
- Vienne A, Hobeika E, Gouya H, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc 2010;72:728-35. [Crossref] [PubMed]
- Bulajic M, Panic N, Radunovic M, et al. Clinical outcome in patients with hilar malignant strictures type II Bismuth-Corlette treated by minimally invasive unilateral versus bilateral endoscopic biliary drainage. Hepatobiliary Pancreat Dis Int 2012;11:209-14. [Crossref] [PubMed]
- Wang AY. Is plastic stenting for pancreatic cancer still relevant or obsolete in 2015? Gastrointest Endosc 2015;81:367-9. [Crossref] [PubMed]
- Lamarca A, Rigby C, McNamara MG, et al. Impact of biliary stent-related events in patients diagnosed with advanced pancreatobiliary tumours receiving palliative chemotherapy. World J Gastroenterol 2016;22:6065-75. [Crossref] [PubMed]
- Linder S, Bostrom L, Nilsson B. Pancreatic cancer in Sweden 1980-2000: a population-based study of hospitalized patients concerning time trends in curative surgery and other interventional therapies. J Gastrointest Surg 2006;10:672-8. [Crossref] [PubMed]
- Speer AG, Cotton PB, Russell RC, et al. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet 1987;2:57-62. [Crossref] [PubMed]
- Baniya R, Upadhaya S, Madala S, et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage after failed endoscopic retrograde cholangiopancreatography: a meta-analysis. Clin Exp Gastroenterol 2017;10:67-74. [Crossref] [PubMed]
- Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy 2001;33:898-900. [Crossref] [PubMed]
- Burmester E, Niehaus J, Leineweber T, et al. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc 2003;57:246-51. [Crossref] [PubMed]
- Giovannini M, Dotti M, Bories E, et al. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy 2003;35:1076-8. [Crossref] [PubMed]
- Shah SL, Perez-Miranda M, Kahaleh M, et al. Updates in Therapeutic Endoscopic Ultrasonography. J Clin Gastroenterol 2018;52:765-72. [PubMed]
- Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: a systematic review. Gastrointest Endosc 2016;83:1218-27. [Crossref] [PubMed]
- Dhir V, Bhandari S, Bapat M, et al. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointest Endosc 2012;75:354-9. [Crossref] [PubMed]
- Paik WH, Lee TH, Park DH, et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol 2018;113:987-97. [Crossref] [PubMed]
- Park JK, Woo YS, Noh DH, et al. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc 2018;88:277-82. [Crossref] [PubMed]
- Bang JY, Navaneethan U, Hasan M, et al. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc 2018;88:9-17. [Crossref] [PubMed]
- Tonozuka R, Itoi T, Tsuchiya T, et al. EUS-guided biliary drainage is infrequently used even in high-volume centers of interventional EUS. Gastrointest Endosc 2016;84:206-7. [Crossref] [PubMed]
- Holt BA, Hawes R, Hasan M, et al. Biliary drainage: role of EUS guidance. Gastrointest Endosc 2016;83:160-5. [Crossref] [PubMed]
- Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol 2017;10:42-53. [Crossref] [PubMed]
- Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: cumulative experience of 101 procedures at a single center. Endoscopy 2015;47:794-801. [Crossref] [PubMed]
- Khan MA, Akbar A, Baron TH, et al. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig Dis Sci 2016;61:684-703. [Crossref] [PubMed]
- Uemura S, Iwashita T, Iwata K, et al. Endoscopic duodenal stent versus surgical gastrojejunostomy for gastric outlet obstruction in patients with advanced pancreatic cancer. Pancreatology 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Ardengh JC, Lopes CV, Kemp R, et al. Different options of endosonography-guided biliary drainage after endoscopic retrograde cholangio-pancreatography failure. World J Gastrointest Endosc 2018;10:99-108. [Crossref] [PubMed]
- Ogura T, Higuchi K. Technical tips for endoscopic ultrasound-guided hepaticogastrostomy. World J Gastroenterol 2016;22:3945-51. [Crossref] [PubMed]
- Cho DH, Lee SS, Oh D, et al. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos) Gastrointest Endosc 2017;85:1067-75. [Crossref] [PubMed]
- De Cassan C, Bories E, Pesenti C, et al. Use of partially covered and uncovered metallic prosthesis for endoscopic ultrasound-guided hepaticogastrostomy: Results of a retrospective monocentric study. Endosc Ultrasound 2017;6:329-35. [Crossref] [PubMed]
- Stier MW, Waxman I. Lumen-Apposing Metal Stents: Which One and Why? Gastrointest Endosc Clin N Am 2018;28:207-17. [Crossref] [PubMed]
- Dawod E, Kahaleh M. Management of Benign and Malignant Pancreatic Duct Strictures. Clin Endosc 2018;51:156-60. [Crossref] [PubMed]
- Löhr JM, Dominguez-Munoz E, Rosendahl J, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU) United European Gastroenterol J 2017;5:153-99. [Crossref] [PubMed]
- Costamagna G, Gabbrielli A, Mutignani M, et al. Treatment of "obstructive" pain by endoscopic drainage in patients with pancreatic head carcinoma. Gastrointest Endosc 1993;39:774-7. [Crossref] [PubMed]
- Tham TC, Lichtenstein DR, Vandervoort J, et al. Pancreatic duct stents for “obstructive type” pain in pancreatic malignancy. Am J Gastroenterol 2000;95:956-60. [PubMed]
- Costamagna G, Mutignani M. Pancreatic stenting for malignant ductal obstruction. Dig Liver Dis 2004;36:635-8. [Crossref] [PubMed]
- Wehrmann T, Riphaus A, Frenz MB, et al. Endoscopic pancreatic duct stenting for relief of pancreatic cancer pain. Eur J Gastroenterol Hepatol 2005;17:1395-400. [Crossref] [PubMed]
- Fujii-Lau LL, Levy MJ. Endoscopic ultrasound-guided pancreatic duct drainage. J Hepatobiliary Pancreat Sci 2015;22:51-7. [Crossref] [PubMed]
- Kamata K, Takenaka M, Minaga K, et al. EUS-Guided Pancreatic Duct Drainage for Repeat Pancreatitis in a Patient with Pancreatic Cancer. Oncology 2017;93 Suppl 1:87-8. [Crossref] [PubMed]
- Tsauo J, Yoo MW, Song HY, et al. Partially-covered stent placement versus surgical gastrojejunostomy for the palliation of malignant gastroduodenal obstruction secondary to pancreatic cancer. Abdom Radiol (NY) 2016;41:2233-40. [Crossref] [PubMed]
- Yoshida Y, Fukutomi A, Tanaka M, et al. Gastrojejunostomy versus duodenal stent placement for gastric outlet obstruction in patients with unresectable pancreatic cancer. Pancreatology 2017;17:983-9. [Crossref] [PubMed]
- Yamao K, Kitano M, Takenaka M, et al. Outcomes of endoscopic biliary drainage in pancreatic cancer patients with an indwelling gastroduodenal stent: a multicenter cohort study in West Japan. Gastrointest Endosc 2018;88:66-75.e2. [Crossref] [PubMed]
- Staub J, Siddiqui A, Taylor LJ, et al. ERCP performed through previously placed duodenal stents: a multicenter retrospective study of outcomes and adverse events. Gastrointest Endosc 2018;87:1499-504. [Crossref] [PubMed]
- Ogura T, Chiba Y, Masuda D, et al. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy 2016;48:163. [PubMed]
- Fritscher-Ravens A, Mosse CA, Mills TN, et al. A through-the-scope device for suturing and tissue approximation under EUS control. Gastrointest Endosc 2002;56:737-42. [Crossref] [PubMed]
- Chopita N, Vaillaverde A, Cope C, et al. Endoscopic gastroenteric anastomosis using magnets. Endoscopy 2005;37:313-7. [Crossref] [PubMed]
- Rimbas M, Larghi A, Costamagna G. Endoscopic ultrasound-guided gastroenterostomy: Are we ready for prime time? Endosc Ultrasound 2017;6:235-40. [Crossref] [PubMed]
- Perez-Miranda M, Tyberg A, Poletto D, et al. EUS-guided Gastrojejunostomy Versus Laparoscopic Gastrojejunostomy: An International Collaborative Study. J Clin Gastroenterol 2017;51:896-9. [Crossref] [PubMed]
- Khashab MA, Bukhari M, Baron TH, et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open 2017;5:E275-E281. [Crossref] [PubMed]
Cite this article as: Waldthaler A, Rutkowski W, Valente R, Arnelo U, Löhr JM. Palliative therapy in pancreatic cancer—interventional treatment with stents. Transl Gastroenterol Hepatol 2019;4:7.