
Histology driven systemic therapy of liposarcoma—ready for prime time?
Liposarcoma is a malignancy that was first described by Rudolf Virchow in 1857 in a patient with a tumor of the thigh (1). Soft tissue sarcomas include many subtypes, including liposarcomas, and are anticipated to have an incidence of 13,040 in the US in 2018 (2). Liposarcomas are uncommon comprising roughly 17% of soft tissue sarcomas. Peak incidence is between 40 and 60 years old, and there is a slight male predominance. More than 50% of liposarcomas occur in the thigh as painless, indolent masses (3). Roughly 1/3 of liposarcomas arise from the retroperitoneum where they can grow to massive size due to few clinical signs and their slow growth rate (4). Stout, in 1944, classified the liposarcomas into histologic subgroups (5). The gross appearance of liposarcomas range from the myxoid liposarcomas (MLPS)—“slimy greyish white tumours bearing some resemblance to a true myxoma” to “yellow masses which may contain firmer areas of paler colour”. An early (1970) case series of 60 patients with liposarcoma revealed a 10% occurrence of pyrexia that remitted with resection of the tumors. Of these 6 patients, 4 had pleomorphic tumors, 1 lipoma like and 1 was myxoid. 5 of the 6 febrile patients died of their disease (6). Fevers are no longer considered a hallmark of liposarcomas.
Overall survival
Determining the overall survival of patients with soft tissue sarcomas can be challenging due to the complexity of the disease, the range of modalities used to treat it and hence, the distributed nature of records. A 2018 high quality retrospective review lends important data to our knowledge base. A total of 130 physicians, with experience treating soft tissue sarcomas, across Europe were recruited to abstract data from medical records. Charts of 805 patients from the United Kingdom, Spain, Germany and France, who were diagnosed with soft tissue sarcomas for which no surgical or radiation options remained, were reviewed. Patients with liposarcoma (n=105) received first line chemotherapy with doxorubicin alone (61.8%), doxorubicin and ifosfamide (14.3%), docetaxel and gemcitabine (2.9%), docetaxel alone (2.9%) and trabectedin (2.9%). Responses were deemed complete response (CR) (3.8%), partial response (PR) (32.4%), stable disease (30.5%) and progressive disease (30.5%). The median overall survival was 16.3 months [95% confidence interval (CI): 13.3–21.0] (7). Whether or not chemotherapy impacts overall survival in patients with soft tissue sarcoma remains an open question. A 2018 publication reported on 150 patients with high risk, resected soft tissue sarcomas. High risk was defined as high grade (2 or 3), large size (≥8 cm), vascular invasion or infiltrative growth and invasion pattern. All patients were treated with 6 cycles of adjuvant doxorubicin and ifosfamide, with radiation between cycles 3 and 4. The 5-year overall survival was 76.1% which compared favorably with historical controls which had a 50% 5-year overall survival rate with adjuvant radiation alone (8). This trial is suggestive, but not conclusive, for the survival benefit of adjuvant chemotherapy. A retrospective analysis of 16,370 patients with stage III soft tissue sarcoma treated between 1998 and 2012 in the United States was performed on the National Cancer Data Base (NCDB). There was a statistically significant (P<0.001) superior overall survival for those receiving chemotherapy, compared with those who did not receive chemotherapy (82.7 versus 51.3 months). The benefit was most pronounced in pleiomorphic undifferentiated sarcoma patients (9).
Histologic subtypes
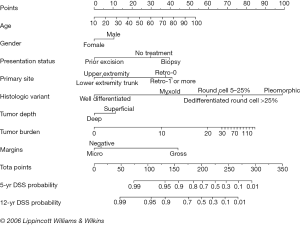
Histologic subtypes of liposarcoma have been described, but there has been variability in the terms used. We now commonly describe four subtypes—well differentiated liposarcoma (WDLPS), dedifferentiated liposarcoma (DDLPS), MLPS and pleomorphic liposarcoma (PLPS). Generally, the myxoid and well differentiated behave as low-grade tumors, with the pleomorphic and dedifferentiated demonstrating more aggressive, high-grade behaviors. Memorial Sloan Kettering Cancer Center has reported the largest series. This was a retrospective review of prospectively collected data for all patients with liposarcoma treated at their institution between 1982 and 2005. They observed a predilection of myxoid, round cell and pleomorphic tumors for the extremities, and well-differentiated and dedifferentiated subtypes for the retroperitoneum. Overall, 910 patients underwent resection during that period, and 801 were evaluable. None of the patients had metastases at the time of presentation. The subtypes seen included 46.2% WDLPS, 17.9% DDLPS, 18.0% MLPS, 10.0% round cell and 8.0% PLPS. Of these tumors, 56.5% were extremity primaries, and 33.5% were retroperitoneal. Overall, survival data varied by tumor subtype with 5-year disease specific (93% WDLPS, 44% DLPS, 92% MLPS, 74% round cell and 59% PLPS) and 12-year disease specific survival (78% WDLPS, 38% DLPS, 86% MLPS, 55% round cell and 53% PLPS) rates. These data have been used to construct prognostic nomograms for not only histologic subtype, but also primary location, and margin status (10) (Figure 1).
Molecular changes
MLPS usually carries a translocation of t(12;16) (q13;p11) involving the DDIT3 and FUS genes (11). A fusion of EWSR1-DDIT3 has been described less commonly. MLPS tumors often express high levels of NY-ESO-1, an immunogenic cancer testis antigen that is a potential target for vaccine-based therapies (12). WDLPS and DDLPS comprise roughly 65% of liposarcomas. Clinically, they can demonstrate late recurrences following surgical resection. The use of molecular diagnostic techniques is better characterizing these groups.
There is a high prevalence of amplification of the 12q14-15 region in WDLPS and DDLPS tumors (Figure 2). This contains the p53 and CDK4 genes which may translate to therapeutic differences with the use of drugs such as palbociclib and abemaciclib. Similarly, MDM2 targeting agents are now in development. RG7112 is a drug developed to inhibit MDM2 (14). Other targets under study in the WDLPS/DDLPS subtype include CRM1—the chromosome region maintenance 1 protein, insulin growth factor-1, MET and PDGFRB.

Location
Patterns of recurrence in retroperitoneal sarcomas vary by subtype. In a review of 675 patients with retroperitoneal sarcomas that underwent surgical exploration at Memorial Sloan Kettering Cancer from July 1982 to July 2010, liposarcomas were separated into a high-grade group (DDLPS, round cell and PLPS) and a low-grade group (WDLPS and MLPS). Totally, 85% of patients were able to have a total gross excision, 9% had grossly positive margins and 6% underwent an exploratory laparotomy but not a resection. The disease specific death (DSD) rate was 25% at 10 years for the low risk group, and 53% for the high-risk group. The high-grade liposarcoma local recurrence rate was 58% by 5 years and 62% at 15 years demonstrating a preponderance of early, local recurrences. In contrast, the low-grade liposarcomas had a 35% local recurrence rate at 5 years with a 60% cumulative rate at 15 years. Distant metastatic disease was present at 10 years in 58% of high-grade liposarcoma patients and 15% in low grade liposarcoma patients (15).
A historical view of chemotherapy for liposarcoma
A report of 88 patients with liposarcomas who received chemotherapy between September 1979 and June 2004 who were treated at the Royal Marsden Hospital were reviewed retrospectively, though the data was captured prospectively. Patients analyzed had received chemotherapy for recurrent or metastatic disease, not in the adjuvant setting. In this evaluation, there were 27 myxoid, 13 round cell, 16 well-differentiated, 16 dedifferentiated, 15 pleomorphic and 1 unspecified liposarcoma. MLPSs demonstrated the highest response rate with 48% achieving a PR to first line chemotherapy compared with 18% of non-MLPSs. The response rate for combined well-differentiated and DDLPSs was only 11%. The number of patients was too small to analyze the response rate to different chemotherapy regimens by histologic subtype. Of the myxoid patients, 5 of 7 patients treated with Doxorubicin responded as died 7 of the 12 patients treated with doxorubicin and ifosfamide (16).
The value of adjuvant or neoadjuvant therapy for sarcoma is tied not only to histologic subtype, but also to location. Retroperitoneal sarcomas are usually identified at large size. In a 1981 trial, Doxorubicin was administered neoadjuvantly to 10 patients (intra-arterially in 2 and intravenously in 8) at UCLA. Four patients also received radiation. Three patients had significant shrinkage pre-operatively, though the authors did not provide details of which therapies the responders underwent, or the doses of either chemotherapy or radiation that were delivered. This added to the conversation around adjuvant and neoadjuvant therapy but did not distinguish among sarcoma types (17).
Unlike many other solid tumors, sarcoma is not generally acknowledged to be highly responsive to chemotherapy. A 1972 report in the Proceedings of the National Cancer Conference reviewed 91 patients treated at Henry Ford Hospital between January 1963 and July 1972 who had metastatic soft tissue sarcomas deemed “impossible to treat” by surgery or radiation. This included 7 patients with liposarcoma. Four of which were retroperitoneal. There was 1 PR in this group—a 14-year-old girl responded to cyclophosphamide and vincristine, and a 52-year-old man had stable disease for over a year to actinomycin D. Histologic subtypes of these tumors were not reported (18). In 1973, an editorial in the journal Surgery, Gynecology and Obstetrics stated “None of the currently used chemotherapeutic agents, to my knowledge, is effective in the management of primary soft tissue sarcomas in adults.” (19).
Doxorubicin, ifosfamide, gemcitabine, docetaxel, trabectedin and eribulin are now considered to be some of the most active agents in the treatment of soft tissue sarcomas in general, and liposarcoma in specific. The limitation of available data related to the subtypes’ responsiveness to different agents is reflected in the absence of such discussions in review articles about these agents. A 2015 systematic review of gemcitabine-based regimens in sarcoma did not reference the histologic subtypes of liposarcoma (20).
Anthracyclines are some of the first drugs to demonstrate meaningful efficacy in soft tissue sarcoma. In a 1972 report from the Southwest Cancer Chemotherapy Study Group, Daunomycin was used in progressive metastatic soft tissue sarcomas in children, and objective tumor shrinkage was seen in 4/21 treated patients. Only one of the patients had a liposarcoma—described as a lipomyxosarcoma—and that patient did not respond (21). A review of 2,185 patients with advanced (relapsed, unresectable or metastatic) soft tissue sarcoma who were treated on 7 studies conducted by the Soft Tissue and Bone Sarcoma Group (STBSG) of the European Organization for Research and Treatment of Cancer (EORTC) were reviewed. These trials included doxorubicin and epirubicin based regimens. Overall survival times were indistinguishable across the regimens, and the overall response rate was 26% with considerable, but statistically insignificant, variation across treatment arms. In this analysis, liposarcoma patients had a statistically significantly superior median overall survival (76 vs. 51 weeks, P=0.0005) (22).
Do different subtypes of liposarcoma respond differently to chemotherapy?
There is good evidence that histologic subtypes of sarcoma respond differently to distinct regimens of chemotherapy (23). A case report in 1983 described the dramatic response of a patient with a poorly differentiated MLPS of the retroperitoneum to treatment with prednisolone, cyclophosphamide, vincristine and actinomycin D (24). In 1994 a study of sensitivity of subtypes of sarcomas using the succinate dehydrogenase inhibition (SDI) test was reported. This chemosensitivity assay was based on the reduction in succinate dehydrogenase activity (SDI) in drug exposed cells to less than 50% of that in control cells. This reduction serves as a surrogate marker of cell death. This assay demonstrated sensitivity of leiomyosarcoma for doxorubicin, and for liposarcoma to cisplatin and aclacinomycin A (an early anthracycline) (25).
The combination of Gemcitabine and Docetaxel is effective in leiomyosarcoma but not in MLPS. A phase II trial of this combination in 44 eligible patients with leiomyosarcoma demonstrated a 25% PR and 36.6% stable disease rate (26). In contrast, doxorubicin combined with ifosfamide demonstrated a 43.2% overall response rate in a retrospective study of 27 patients with MLPS (27).
MD Anderson reported on 44 patients with MLPS presented at the multidisciplinary planning conference between 1986 and 1992. A total of 21 of these patients received systemic chemotherapy, 20 were evaluable and of these, 18 received doxorubicin as a continuous infusion with dacarbazine with or without cyclophosphamide. There were 8 objective responses (1 complete) for a response rate of 44% (28). A subsequent report from MD Anderson described 37 chemotherapy naive patients with MLPS who were treated between 1/00 and 12/09. These patients received up to 8 cycles of doxorubicin and ifosfamide. The response rate of 43% was the same for locally advanced and metastatic disease (27).
Studies of newer agents give greater insight into differential response rates due to more stringent attention to tissue subtypes. Trabectedin gained Food and Drug Administration (FDA) approval for leiomyosarcomas and liposarcomas in 2015. Expanded access to trabectedin was evaluated for 1,895 patients. Only 807 were assessable. Clinical benefit [CR, PR or stable disease] was seen in 54% of the 258 patients with either leiomyosarcoma or liposarcoma, compared with 38% of patients with other soft tissue sarcomas (29,30). The MLPSs appears to be particularly sensitive to trabectedin. The mechanism has been proposed to be functional inactivation of the oncogenic chimera FUS-CHOP, with a downstream impact on adipocyte differentiation. The authors studying this mechanism commented that “To our knowledge this is the first report indicating that a small molecule can displace an oncogenic transcription factor in vivo from its target DNA sequences, thus specifically modulating the transcription of genes involved in the pathogenesis of a neoplastic disease.” (31).
Aldoxorubicin is an albumin bound doxorubicin prodrug that was compared with doxorubicin in a phase II trial of patients with advanced soft tissue sarcomas. Despite improvement in median progression free survival (PFS) (5.6 vs. 2.7 months, P=0.02) and response rate (25% vs. 0%), there was no improvement in median overall survival (15.8 vs. 14.3 months) (32). A subsequent phase III clinical trial compared aldoxorubicin with physician’s choice chemotherapy. There were no differences in overall survival for the group as a whole, but the liposarcoma subset had prolonged median PFS (5.3 vs. 3.0 months, P=0.007). There was no commentary on specific liposarcoma subtypes (33).
Olaratumab (a human anti-PDGFR alpha monoclonal antibody) was studied in a randomized trial as a combination therapy with doxorubicin versus doxorubicin alone in patients with anthracycline naive advanced soft tissue sarcoma. In this phase Ib/II study, median survival was 12 months longer in the combined therapy arm (26.5 vs. 14.7 months, P=0.0003). Interestingly, objective response rate and PFS did not differ between the two groups. No characterization of liposarcoma subtypes was made (34). The results of a phase III trial as first line treatment has completed accrual but results have not yet been published.
When considering the variability of sarcomas response to therapy, it is important to consider why this might be. The data from anti-angiogenesis agents gives an important clue. There is a difference in the level of platelet-derived growth factor receptors (PDGFRA and PDGFRB), and vascular endothelial growth factor receptors (VEGFR-1 to VEGFR-3) in different subtypes of soft tissue sarcoma. Pazopanib and sunitinib are tyrosine kinase inhibitors that act on these targets, and histologic subtypes of sarcomas have a broad range of response rates (35). Early studies of pazopanib demonstrated a clinically and statistically significant decreased efficacy in the liposarcoma subset of soft tissue sarcomas. For that reason, liposarcomas are routinely excluded from further trials of pazopanib (36).
Regorafenib is an oral tyrosine kinase inhibitor that binds to and inhibits VEGFRs 2 and 3, as well as, Ret, Kit, PDGFR and Raf. This agent was studied in a phase II, randomized trial compared with placebo in four soft tissue sarcoma subtypes (liposarcoma, leiomyosarcoma, synovial sarcoma and mixed histologies). PFS was superior in all subgroups except liposarcoma, and overall survival was superior in the pooled subgroups when liposarcoma was excluded. In the liposarcoma subgroup analysis, PFS was 1.1 month in the regorafenib group compared with 1.7 months with placebo (37).
WDLPS and DDLPS have amplification of CDK4 in 90% of cases. A clinical trial of 60 WDLPS or DDLPS patients treated with palbociclib, an oral CDK4 and CDK6 inhibitor, at the doses approved by the FDA for the treatment of patients with breast cancer—125 mg daily for 21 out of 28 days. The study used PFS as its primary endpoint, and at 12 weeks (PFS12) the rate was 57.2%. There was one CR observed (38).
The original phase II trial of pazopanib in soft tissue sarcomas revealed minimal activity in liposarcomas. The study used progression free rate (PFR) as its primary endpoint, and at 12 weeks (PFR12), this was demonstrated in only 5/19 (26%) of liposarcoma patients compared with 18/41 (44%) of leiomyosarcoma patients, 18/38 (49%) of synovial sarcoma patients and 16/41 (39%) of patients with other soft tissue subtypes (36). On the basis of this difference, patients with liposarcoma have been excluded from subsequent trials of pazopanib. This was revisited in 2017 with the publication of a single arm, phase II trial of pazopanib in advanced liposarcoma patients that demonstrated a PFR12 of 68.3%. More interestingly, at 24 weeks, 39% of patients were progression free and the median overall survival was 12.6 months. In this study, the PFR12 was 74.1% for DDLPS, and 66.7% for myxoid/round cell liposarcoma. The number of patients with PLPSs was felt to be too small to be assessable (39).
In a proof of concept study, 20 patients were enrolled at 4 centers in France and treated with the novel agent RG7112. Totally, 14 of 17 assessable patients with WDLPS or DDLPS expressed amplification of the MDM2 gene, the target of this agent. After a maximum of 4 28-day cycles of this therapy in the neoadjuvant context, 1 PR and 14 stable diseases were observed. Toxicity was significant and predominantly hematologic (13).
The, as yet, limited data on the role of immune checkpoint inhibitors in soft tissue sarcomas overall precludes any comparison among the subtypes of liposarcoma. A single arm trial, SARC28, enrolled a cohort of 10 patients with DDLPS to treatment with Pembrolizumab. Two PRs were observed. Of note, there were 6 patients with soft tissue sarcoma in the overall trial that responded, but only 2 were positive for programmed death ligand 1 (PD-L1) (40). Additional trials are underway exploring combination anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death-1 (PD-1) inhibitors, as well as, the integration of chemo and immunotherapies. It is premature to surmise significant efficacy in soft tissue sarcomas as a whole, and insufficient data exists to allow any suppositions about response rates across the subtypes.
Adjuvant therapy
There is limited reported, detailed information about the response of subtypes of liposarcoma to adjuvant therapy. Zagars et al. reported a <10% local recurrence rate with almost no metastatic disease in patients with WDLPSs treated with radiation following resection, compared with roughly 33% local recurrence and 40% metastases for similarly treated PLPSs (41).
There have been several major studies exploring adjuvant chemotherapy. The Italian Sarcoma Group compared 3 versus 5 cycles of adjuvant epirubicin and ifosfamide in patients with high risk spindle cell sarcoma of the superficial trunk or limbs who had undergone resection ± radiation. This study was stopped early (at a median 59 month follow up) when there was a significant positive improvement in both disease free and overall survival (42). At a later follow up assessment (median 90 month follow up), the improvement due to adjuvant chemotherapy versus no treatment lost statistical significance (43).
Adjuvant chemotherapy for soft tissue sarcoma was evaluated in a randomized study of doxorubicin and ifosfamide versus no therapy in EORTC 62931. This, the largest such trial, in 351 patients with macroscopically resected soft tissue sarcoma, demonstrated no benefit from chemotherapy in terms of local control, relapse free or overall survival (44,45).
Neoadjuvant therapy (treatment prior to surgery) carries the theoretical benefit of treating occult metastases early in the course of therapy, or reducing the extent of required resection. The potential downside to this approach includes impaired wound healing and the possibility that a resectable tumor might grow through ineffective treatment and become unresectable. In patients with surgically resected soft tissue sarcomas, the complication rate for preoperative radiation was 22% compared with 6% for post-operative radiation (46).
The role of adjuvant chemotherapy was studied in a phase three Italian Sarcoma Group trial in patients with high risk superficial trunk or limb soft tissue sarcoma. Patients received 3 neoadjuvant cycles of epirubicin and ifosfamide and either no additional chemotherapy or 2 cycles in the adjuvant setting. Radiation could be given either pre or post operatively. There was no significant difference in 10-year overall survival between the groups (47).
As the importance of histologic subtypes has been increasingly recognized, we are exploring how to incorporate it into treatment decisions. A phase 3 randomized trial of neoadjuvant treatment in patients with high risk, yet resectable, soft tissue sarcoma of the trunk wall or limb, compared 3 cycles of epirubicin and ifosfamide to 3 cycles of chemotherapy determined by histology. Patients also were treated with surgery and radiation. The histology determined systemic therapy included gemcitabine and dacarbazine for leiomyosarcoma, trabectedin for myxoid round cell liposarcoma, high dose ifosfamide for synovial sarcoma, ifosfamide and etoposide for malignant peripheral nerve sheath tumors, and gemcitabine with docetaxel for undifferentiated pleomorphic sarcoma. At a mean follow-up of 12.3 months, the standard chemotherapy was superior to histology driven chemotherapy with a 46-month disease free survival of 62% versus 38% (P=0.006). In myxoid-round cell liposarcoma, however, relapse free survival was similar with trabectedin and the standard epirubicin with ifosfamide. This study included 390 patients and was terminated early. We await further results (48).
Discussion
Subtypes of soft tissue sarcoma have been described for over 150 years. Histologic subclassification of liposarcomas has been utilized for over 70 years. In the last 10–20 years, molecular and genetic characterization of these subtypes has evolved. Although few inclusive trials of systemic therapy have incorporated analysis of the response of histologic subtypes, several agents are being studied in specific subsets. Current clinical trials are underway in DDLPS of the agents cabazitaxel and plitidepsin. MLPSs is being evaluated for their response to sirolimus and cyclophosphamide in combination. In addition, based on the high response rate of MLPSs to chemotherapy, and the frequent presence of NY-ESO-1, T-cell directed cellular therapy targeting that antigen is the subject of a current clinical trial (Clinicaltrials.gov 7/24/18).
In 2018, there is insufficient evidence to recommend selection of systemic therapy on the basis of the histologic subtype in most liposarcomas. An exception is MLPSs, which demonstrate particular sensitivity to trabectedin, though they generally have superior response to other agents as well. Analyses of histologic subtypes’ responsiveness to several newer agents, including Aldoxorubicin and Olaratumab, have not yet been published. A trial of histology driven selection of liposarcoma chemotherapy failed to achieve the desired improvement over a standard regimen. Extension of this concept to the study of histology driven subtypes of liposarcoma would be enlightening. Olaratumab and Aldoxorubicin have not yet been analyzed for their effectiveness in the varied subtypes of liposarcoma.
In an uncommon disease, liposarcoma, the opportunity for study at the subtype level will require a collaborative effort among multiple institutions, and potentially across nations, to have sufficient cohort size for statistical evaluation. For now, the selection of systemic agents in the treatment of liposarcomas is not feasible due to the lack of robust data. The one exception is the myxoid/round cell liposarcoma subgroup. As a community of oncologists, we may choose to make recommendations regarding the inclusion of histologic subtype characterization in therapeutic trials, as a means of enabling subsequent meta-analysis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Virchow R. Ein Fall von bösartigen, zum Theil in der Form des Neuroms auftretenden Fettgeschwülsten. Virchows Arch Pathol Anat Physiol Klin Med 1857;11:281-8. [Crossref]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Coffin CM. Adipose and myxoid tumors. In: Coffin C, LP D, PA OS, editors. Pediatric Soft Tissue Tumors: A Clinical, Pathological and Therapeutic Approach. Baltimore: Williams & Wilkins; 1997:254-76.
- Peterson JJ, Kransdorf MJ, Bancroft LW, et al. Malignant fatty tumors: classification, clinical course, imaging appearance and treatment. Skeletal Radiol 2003;32:493-503. [Crossref] [PubMed]
- Stout AP. Liposarcoma—the Malignant Tumor of Lipoblasts*. Ann Surg 1944;119:86-107. [PubMed]
- Spittle MF, Newton KA, Mackenzie DH. Liposarcoma. A review of 60 cases. Br J Cancer 1970;24:696-704. [Crossref] [PubMed]
- Nagar SP, Mytelka DS, Candrilli SD, et al. Treatment Patterns and Survival among Adult Patients with Advanced Soft Tissue Sarcoma: A Retrospective Medical Record Review in the United Kingdom, Spain, Germany, and France. Sarcoma 2018;2018:5467057. [Crossref] [PubMed]
- Sundby Hall K, Bruland OS, Bjerkehagen B, et al. Adjuvant chemotherapy and postoperative radiotherapy in high-risk soft tissue sarcoma patients defined by biological risk factors-A Scandinavian Sarcoma Group study (SSG XX). Eur J Cancer 2018;99:78-85. [Crossref] [PubMed]
- Movva S, von Mehren M, Ross EA, et al. Patterns of Chemotherapy Administration in High-Risk Soft Tissue Sarcoma and Impact on Overall Survival. J Natl Compr Canc Netw 2015;13:1366-74. [Crossref] [PubMed]
- Dalal KM, Kattan MW, Antonescu CR, et al. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg 2006;244:381-91. [PubMed]
- Fujii Y, Matui Y, Nakagawa Y, et al. Translocation t(12;16)(q13;p11) in myxoid liposarcoma of a child and implication of the human int-1 gene in tumorigenesis. Jpn J Cancer Res 1989;80:958-62. [Crossref] [PubMed]
- Pollack SM, Jungbluth AA, Hoch BL, et al. NY-ESO-1 is a ubiquitous immunotherapeutic target antigen for patients with myxoid/round cell liposarcoma. Cancer 2012;118:4564-70. [Crossref] [PubMed]
- Arici A, Ozgur T, Ugras N, et al. Immunohistochemical detection of p53 and MDM2 expressions in liposarcoma with World health organization classification. Indian J Cancer 2013;50:164-9. [Crossref] [PubMed]
- Ray-Coquard I, Blay JY, Italiano A, et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol 2012;13:1133-40. [Crossref] [PubMed]
- Tan MC, Brennan MF, Kuk D, et al. Histology-based Classification Predicts Pattern of Recurrence and Improves Risk Stratification in Primary Retroperitoneal Sarcoma. Ann Surg 2016;263:593-600. [Crossref] [PubMed]
- Jones RL, Fisher C, Al-Muderis O, et al. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer 2005;41:2853-60. [Crossref] [PubMed]
- Storm FK, Eilber FR, Mirra J, et al. Retroperitoneal sarcomas: a reappraisal of treatment. J Surg Oncol 1981;17:1-7. [Crossref] [PubMed]
- Talley RW. Chemotherapy of soft-tissue sarcomas. Proc Natl Cancer Conf 1972;7:89-900. [PubMed]
- Das Gupta TK. Editorial: Management of soft tissue sarcomas. Surg Gynecol Obstet 1973;137:1012-3. [PubMed]
- Ducoulombier A, Cousin S, Kotecki N, et al. Gemcitabine-based chemotherapy in sarcomas: A systematic review of published trials. Crit Rev Oncol Hematol 2016;98:73-80. [Crossref] [PubMed]
- Sutow WW, Vietti TJ, Lonsdale D, et al. Daunomycin in the treatment of metastatic soft tissue sarcoma in children. Cancer 1972;29:1293-7. [Crossref] [PubMed]
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 1999;17:150-7. [Crossref] [PubMed]
- Katz D, Palmerini E, Pollack SM. More Than 50 Subtypes of Soft Tissue Sarcoma: Paving the Path for Histology-Driven Treatments. Am Soc Clin Oncol Educ Book 2018.925-38. [Crossref] [PubMed]
- Ogihara T, Inagaki Y, Nagata S, et al. Combination chemotherapy in retroperitoneal poorly differentiated myxoid liposarcoma: a report of a case. Jpn J Med 1983;22:37-9. [Crossref] [PubMed]
- Takeuchi H, Baba H, Inutsuka S, et al. Antitumor Chemosensitivity Differs between Clinical Sarcoma and Adenocarcinoma Tissues. Anticancer Res 1994;14:169-71. [PubMed]
- Seddon B, Scurr M, Jones RL, et al. A phase II trial to assess the activity of gemcitabine and docetaxel as first line chemotherapy treatment in patients with unresectable leiomyosarcoma. Clin Sarcoma Res 2015;5:13. [Crossref] [PubMed]
- Katz D, Boonsirikamchai P, Choi H, et al. Efficacy of first-line doxorubicin and ifosfamide in myxoid liposarcoma. Clin Sarcoma Res 2012;2:2. [Crossref] [PubMed]
- Patel SR, Burgess MA, Plager C, et al. Myxoid liposarcoma. Experience with chemotherapy. Cancer 1994;74:1265-9. [Crossref] [PubMed]
- Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol 2009;27:4188-96. [Crossref] [PubMed]
- Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703-9. [Crossref] [PubMed]
- Di Giandomenico S, Frapolli R, Bello E, et al. Mode of action of trabectedin in myxoid liposarcomas. Oncogene 2014;33:5201-10. [Crossref] [PubMed]
- Chawla SP, Papai Z, Mukhametshina G, et al. First-Line Aldoxorubicin vs Doxorubicin in Metastatic or Locally Advanced Unresectable Soft-Tissue Sarcoma: A Phase 2b Randomized Clinical Trial. JAMA Oncol 2015;1:1272-80. [Crossref] [PubMed]
- Chawla SP, Ganjoo KN, Schuetze S, et al. Phase III study of aldoxorubicin vs investigators’ choice as treatment for relapsed/refractory soft tissue sarcomas. J Clin Oncol 2017;35:11000. [Crossref]
- Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488-97. [Crossref] [PubMed]
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879-86. [Crossref] [PubMed]
- Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126-32. [Crossref] [PubMed]
- Mir O, Brodowicz T, Italiano A, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016;17:1732-42. [Crossref] [PubMed]
- Dickson MA, Schwartz GK, Keohan ML, et al. Progression-Free Survival Among Patients With Well-Differentiated or Dedifferentiated Liposarcoma Treated With CDK4 Inhibitor Palbociclib: A Phase 2 Clinical Trial. JAMA Oncol 2016;2:937-40. [Crossref] [PubMed]
- Samuels BL, Chawla SP, Somaiah N, et al. Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Cancer 2017;123:4640-7. [Crossref] [PubMed]
- Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 2017;18:1493-501. [Crossref] [PubMed]
- Zagars GK, Goswitz MS, Pollack A. Liposarcoma: outcome and prognostic factors following conservation surgery and radiation therapy. Int J Radiat Oncol Biol Phys 1996;36:311-9. [Crossref] [PubMed]
- Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850-6. [Crossref] [PubMed]
- Gronchi A, Stacchiotti S, Verderio P, et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): long-term follow-up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann Oncol 2016;27:2283-8. [Crossref] [PubMed]
- Dangoor A, Seddon B, Gerrand C, et al. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res 2016;6:20. [Crossref] [PubMed]
- Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 2012;13:1045-54. [Crossref] [PubMed]
- Pollack A, Zagars GK, Goswitz MS, et al. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. Int J Radiat Oncol Biol Phys 1998;42:563-72. [Crossref] [PubMed]
- Palassini E, Ferrari S, Verderio P, et al. Feasibility of Preoperative Chemotherapy With or Without Radiation Therapy in Localized Soft Tissue Sarcomas of Limbs and Superficial Trunk in the Italian Sarcoma Group/Grupo Espanol de Investigacion en Sarcomas Randomized Clinical Trial: Three Versus Five Cycles of Full-Dose Epirubicin Plus Ifosfamide. J Clin Oncol 2015;33:3628-34. [Crossref] [PubMed]
- Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18:812-22. [Crossref] [PubMed]
Cite this article as: Grethlein SJ. Histology driven systemic therapy of liposarcoma—ready for prime time? Transl Gastroenterol Hepatol 2018;3:96.


