Translational medical research and liver transplantation: systematic review
Introduction
Translational medicine aims to introduce innovations from basic research to clinical development and has become a priority. Nevertheless, there is still a vast challenge to the arrival of new treatments and their real benefits for practical medicine (1). Indeed, advances are not sustained by the absence of basic research, and therefore strategies and a methodology have been shaped in order to develop new treatment, mainly for complex diseases that demand better resolutions (1).
Liver transplantation (LT) is an efficacy approach and complex therapeutic technique in medicine. Despite of all basic research accomplished for this therapeutic method, this procedure still has many complications that require scientific models in order to improve the results (2). Additionally, promoting the validation of studies in precision medicine, immunological and surgical fields (including genome and epigenome studies), nanotechnology, signaling pathways, and biobanking could improve the advances of translational research and personalized medicine toward LT, offering potential solutions for further advancement through better integration between health care, academia, and industry (3).
The purpose of this literature systematic review was to analyze the translational process in the specific setting of LT, including liver ischemia-reperfusion injury (IRI), immunosuppression, clinical and surgical complications, small-for-size syndrome (SFSS), rejection, and ongoing innovations (liver machine, liver preservation, artificial livers, and regenerative medicine).
Methods
Identification and selection of the studies
This literature systematic review was performed to up to date the translational medical research in LT. The Medline-PubMed, Embase, Cochrane Databases (Controlled Trials and systematic review) and LILACS databases were electronically searched for articles published from January 2000 to October 2016, and updated in 19 October 2016.
The Mesh-terms utilized in Medline-PubMed database for literature search were developed using the PICO structure: patient, intervention, comparison or control, outcome. The Mesh-terms were used in combination with “OR”. The results for the search Mesh-terms used “P” (Patients) were associated with the result that formed with the “I” (Intervention), using the “AND” and “NOT” operators.
The Medline-PubMed search was conducted in PubMed (www.ncbi.nlm.nih.gov/pubmed) and used the Mesh-terms: (((((“Liver Transplantation”[Mesh]) AND “Translational Medical Research”[Mesh]) and (((((“Liver Transplantation”[Mesh]) AND “Translational Medical Research”[Mesh]) OR “Genomics”[Mesh]) OR “Epigenomics”[Mesh]) OR “Molecular Targeted Therapy”[Mesh]) OR “Bioethics”[Mesh])))). The equal strategy was used the others databases in the Embase (www.embase.com) and LILACS (http://lilacs.bvsalud.org) with the terms: (((((“Liver Transplantation”[Mesh]) AND “Translational Medical Research”[Mesh]). The Cochrane Library Database (http://www.cochrane.org) was searched for registered and published systematic reviews (CDSR) and clinical trials (CCTR) on the management of LT and Translational Medical Research.
Inclusion and exclusion criteria
Selection criteria were performed within the research question with the PICO structure; therefore, randomized controlled trials, non-randomized controlled trials, or comparative clinical studies and others were included. Specific analysis and article selection in the LT surgical approach include the following topics: liver IRI; immunosuppression; complications: clinical and surgical; SFSS; rejection; and miscellaneous (liver machine and liver preservation; artificial liver and regenerative medicine; and experimental LT model).
Data collection, analysis, and critical evaluation
Independently the reviewers were assessed the studies quality and extracted data. The quality and selection of the studies data was evaluated by 2 researchers (LS Nacif and V Kim). In case of discordance, the researchers promoted a consensus to select the final verdict. The study design and quality of the studies, level of evidence, and article choice were based on the article’s close relation to the topic of this review.
Results
Study selection and study quality
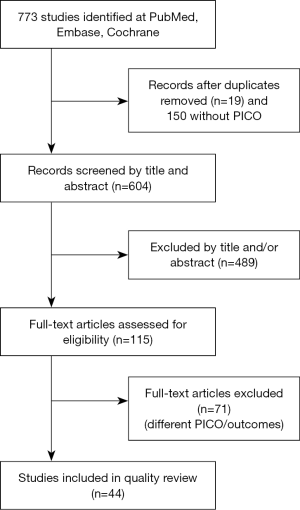
Initially the search found 773 articles. The entire study selection and description are detailed in Figure 1.
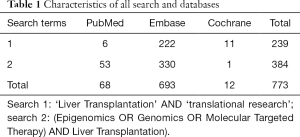
The characteristics of all search and databases were demonstrated on Table 1. Cochrane Library Database (http://www.cochrane.org) literature was searched for registered and published systematic reviews (CDSR) and clinical trials (CCTR) on the management of LT and Translational Medical Research (Table 1).
Full table
Articles selection with PICO structure were summarized in Supplementary files.
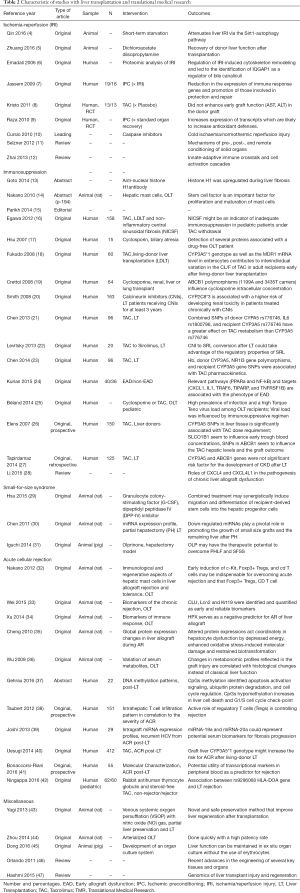
In the qualitative analysis of the present study we selected 44 studies for further analysis (4-23), the specific analysis was demonstrated on Figure 1 and Table 2 (24-47).
Full table
Qualitative analysis evaluation of the selected articles was follow below as liver IRI (n=9); immunosuppression (n=16); SFSS (n=3); acute cellular rejection (ACR) (n=11); liver machine and preservation; artificial livers and regenerative medicine (n=5) (4-23). The sensitive analysis shows us the vast presence of prospective original studies in these fields with more than 33 articles selected, the others was retrospective and reviews. The articles selected in the search were detailed and demonstrated in Table 2 (24-47). The selected articles were initially tabulated and characterized according to study type and PICO structures (Table 2).
Discussion
The present subject of this article shows us the important topic from “bench to bedside” is being re-thought around the world in the scientific and health-care communities. An integrated institutional, multidisciplinary medical and research approach for the application of translational medicine promotes the validation of studies to translate to the clinical and surgical fields regarding LT. Indeed, diverse advances on translational research have been made over the past decades in the field of LT in an attempt to increase organ utilization and improve the health care for these patients.
Liver IRI is an important complication from hemorrhagic shock, large resection, and transplant. IRI in general is a dynamical manner with two stages: the local insult on ischemia phase and reperfusion phase with inflammation mediators (12). Regarding the LT process, these injuries occur at three distinct steps: firstly, the liver is stored at 0 °C to 4 °C after explanted from the donor (cold ischemia); then followed by the vascular anastomosis (warm ischemia). The ischemia period increases the liver temperature 0.5 °C/minute. Liver reperfusion configures the third step (8,10,11). Over the last decade, the prevention of early graft failure was performed using some therapeutic approach and donor-recipient well match strategies to prevent and decrease the IRI effects.
Immunosuppressive therapy has been optimized and evolving over the last years following LT (2). The therapeutic options vary from diverse types of agents and depend on the specific patient whether one should use the immunosuppression agent alone or in association. The types of LT immunosuppression drugs include: calcineurin inhibitors (CNIs), cyclosporine (CsA) or TAC; mycophenolate mofetil (MMF), azathioprine (AZA), mTOR inhibitors (rapamycin), and steroids (methylprednisolone or prednisone) (2). Post-transplant acute or chronic rejection and fibrosis are even difficulties to obtaining long-term survival in LT. Some translational studies show us interesting advances in this field, the mostly studies found in this review was regarding the acute cellular rejection (n=11) and immunosuppression (n=16).
Chronic kidney disease (CKD) after liver transplant is one of the biggest clinical problems that arise to be related with genetic and non-genetic determinants. CNI is considered the main guilty in the development of CKD after LT (15). Kidney injury is usual in recipients with cirrhosis and related with decrease of survival rate after LT. This dysfunction perhaps associated to damage or functional alterations and can be better with following the LT.
Another poorly understood complication in LT is the concept of early allograft dysfunction (EAD), which demonstrates post-transplantation with increase serum transaminases, cholestasis and coagulopathy (28). Chronic liver graft dysfunction is the main morbidity and late graft waste cause after LT.
Small remnant of the liver or a small-for-size liver commonly induces SFSS or post-hepatectomy liver failure (PHLF). An inordinate portal flow for these small livers is primordial factor for SFSS (45). Some of these modalities, as living donor LT or split livers, were solutions to the liver donor shortages and decrease the waiting-list time for the patients to the LT procedure (45). This study shows us some specific papers on raising the mortality rate and require the progress of organ culture system that livers can be cultured and hold ex situ for a long time.
Regenerative medicine has demonstrated feasible for “bench-to-bedside” translational research in cell progress, stem cell biology and tissue engineering (46). We found some papers regarding models of functioning livers that have been engineered application “natural tissue” scaffolds and are in evolution to produce kidneys, pancreas, and small intestines.
The regenerative potential of the liver is unparalleled with its regeneration recover after damage from ischemia, resection, and acute or chronic rejection (47). The signatures of gene expression were characterized for these events and may be possible to precise therapies to decrease damage, improve regeneration and survival rates.
The limitations of this study were that we were unable to perform a meta-analysis due to the diversity and heterogeneity of the papers studied. This study has others limitations; the specific outcomes of interest have been evaluated separated. In this subject, more randomized clinical trials are needed to focus on translational medicine and LT. The benefit of this systematic review was to evaluate more patients with important positive factors in the liver transplant field. This is an original research that we described a systematic review of articles that shows us an important topic from “bench to bedside” is being re-thought around the world in the scientific and health-care communities. Indeed, diverse advances on translational research have been made over the past decades in the field of LT in an attempt to increase organ utilization and improve the health care for these patients.
Conclusions
Translational medicine was initially conceived as an integrated institutional, multidisciplinary, medical research approach toward the application of “bench-to-bedside” rather than promoting advancement and technologies in LT around the world in the scientific and health-care communities.
This systematic review demonstrated due to the increasing number of publications that have been improvements regarding the study of translational medicine in LT. Innovative studies and technologies from basic science help to clarify clinical doubts and basic scientific research has increasingly contributed to the quality of care in clinical practice.
Supplementary
Articles selection with PICO structure
- Liver transplantation AND Translational Medical Research
- (Epigenomics OR Genomics OR Molecular Targeted Therapy) AND Liver Transplantation
Duplicates: 5
(“Liver Transplantation”[Mesh]) AND “Translational Medical Research”[Mesh]
Cochrane: 11
(“Liver Transplantation”[Mesh]) AND “Translational Medical Research”[Mesh]
PubMed: 6
‘Liver Transplantation’ AND ‘translational research’
Embase: 222
Duplicates: 14
(((“Epigenomics”[Mesh]) OR “Genomics”[Mesh]) OR “Molecular Targeted Therapy”[Mesh]) AND “Liver Transplantation”[Mesh]
PubMed: 53
(Genomics OR Epigenetics OR molecularly targeted therapy) AND Liver Transplantation
Embase: 330
(Genomics OR Epigenomics OR molecularly targeted therapy) AND Liver Transplantation
Cochrane: 1
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mankoff SP, Brander C, Ferrone S, et al. Lost in Translation: Obstacles to Translational Medicine. J Transl Med 2004;2:14. [Crossref] [PubMed]
- Nacif LS, Pinheiro RS, de Arruda Pécora RA, et al. Re-Transplantation, Higher Creatinine Levels in Hepatitis C Virus Patients, and Donor Age Are Predictors of Mortality in Long-Term Analysis of Late Acute Rejection in Liver Transplantation. Ann Transplant 2017;22:9-16. [Crossref] [PubMed]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793-5. [Crossref] [PubMed]
- Qin J, Zhou J, Dai X, et al. Short-term starvation attenuates liver ischemia-reperfusion injury (IRI) by Sirt1-autophagy signaling in mice. Am J Transl Res 2016;8:3364-75. [PubMed]
- Zhuang Z, Lian P, Wu X, et al. Abate Cytochrome C induced apoptosome to protect donor liver against ischemia reperfusion injury on rat liver transplantation model. Am J Transl Res 2016;8:1738-47. [PubMed]
- Emadali A, Muscatelli-Groux B, Delom F, et al. Proteomic analysis of ischemia-reperfusion injury upon human liver transplantation reveals the protective role of IQGAP1. Mol Cell Proteomics 2006;5:1300-13. [Crossref] [PubMed]
- Jassem W, Fuggle S, Thompson R, et al. Effect of ischemic preconditioning on the genomic response to reperfusion injury in deceased donor liver transplantation. Liver Transpl 2009;15:1750-65. [Crossref] [PubMed]
- Kristo I, Wilflingseder J, Kainz A, et al. Effect of intraportal infusion of tacrolimus on ischaemic reperfusion injury in orthotopic liver transplantation: a randomized controlled trial. Transpl Int 2011;24:912-9. [Crossref] [PubMed]
- Raza A, Dikdan G, Desai KK, et al. Global gene expression profiles of ischemic preconditioning in deceased donor liver transplantation. Liver Transpl 2010;16:588-99. [PubMed]
- Cursio R. Caspase inhibition in liver transplantation: from basic research to clinical studies. HPB (Oxford) 2010;12:1-3. [Crossref] [PubMed]
- Selzner N, Boehnert M, Selzner M. Preconditioning, postconditioning, and remote conditioning in solid organ transplantation: basic mechanisms and translational applications. Transplant Rev (Orlando) 2012;26:115-24. [Crossref] [PubMed]
- Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol 2013;10:79-89. [Crossref] [PubMed]
- Goto S, Hsu L, Nakano T, et al. Significance of post-liver transplantation induced anti-histone H1 antibody for prevention of chronic rejection and liver fibrosis. Transplantation 2014;98:309. [Crossref]
- Nakano T, Goto S, Lai CY, et al. Immunological aspects and therapeutic significance of an autoantibody against histone H1 in a rat model of concanavalin A-induced hepatitis. Immunology 2010;129:547-55. [Crossref] [PubMed]
- Parikh CR, Belcher JM. Reconsidering a "chopped liver": the need for improving glomular filtration rate estimation for hepatic transplantation. Hepatology 2014;59:1242-5. [Crossref] [PubMed]
- Egawa H, Miyagawa-Hayashino A, Haga H, et al. Non-inflammatory centrilobular sinusoidal fibrosis in pediatric liver transplant recipients under tacrolimus withdrawal. Hepatol Res 2012;42:895-903. [Crossref] [PubMed]
- Hsu LW, Goto S, Nakano T, et al. Immunosuppressive activity of serum taken from a liver transplant recipient after withdrawal of immunosuppressants. Transpl Immunol 2007;17:137-46. [Crossref] [PubMed]
- Fukudo M, Yano I, Yoshimura A, et al. Impact of MDR1 and CYP3A5 on the oral clearance of tacrolimus and tacrolimus-related renal dysfunction in adult living-donor liver transplant patients. Pharmacogenet Genomics 2008;18:413-23. [Crossref] [PubMed]
- Crettol S, Venetz JP, Fontana M, et al. Influence of ABCB1 genetic polymorphisms on cyclosporine intracellular concentration in transplant recipients. Pharmacogenet Genomics 2008;18:307-15. [Crossref] [PubMed]
- Smith HE, Jones JP 3rd, Kalhorn TF, et al. Role of cytochrome P450 2C8 and 2J2 genotypes in calcineurin inhibitor-induced chronic kidney disease. Pharmacogenet Genomics 2008;18:943-53. [Crossref] [PubMed]
- Chen D, Fan J, Guo F, et al. Novel single nucleotide polymorphisms in interleukin 6 affect tacrolimus metabolism in liver transplant patients. PLoS One 2013;8:e73405. [Crossref] [PubMed]
- Levitsky J, Mathew JM, Abecassis M, et al. Systemic immunoregulatory and proteogenomic effects of tacrolimus to sirolimus conversion in liver transplant recipients. Hepatology 2013;57:239-48. [Crossref] [PubMed]
- Chen D, Guo F, Shi J, et al. Association of hemoglobin levels, CYP3A5, and NR1I3 gene polymorphisms with tacrolimus pharmacokinetics in liver transplant patients. Drug Metab Pharmacokinet 2014;29:249-53. [Crossref] [PubMed]
- Kurian SM, Fouraschen SM, Langfelder P, et al. Genomic profiles and predictors of early allograft dysfunction after human liver transplantation. Am J Transplant 2015;15:1605-14. [Crossref] [PubMed]
- Béland K, Dore-Nguyen M, Gagné MJ, et al. Torque Teno virus in children who underwent orthotopic liver transplantation: new insights about a common pathogen. J Infect Dis 2014;209:247-54. [Crossref] [PubMed]
- Elens L, Capron A, Kerckhove VV, et al. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharmacogenet Genomics 2007;17:873-83. [Crossref] [PubMed]
- Tapirdamaz Ö, Hesselink DA, el Bouazzaoui S, et al. Genetic variance in ABCB1 and CYP3A5 does not contribute toward the development of chronic kidney disease after liver transplantation. Pharmacogenet Genomics 2014;24:427-35. [Crossref] [PubMed]
- Li J, Liu B, Yan LN, et al. The roles and potential therapeutic implications of CXCL4 and its variant CXCL4L1 in the pathogenesis of chronic liver allograft dysfunction. Cytokine Growth Factor Rev 2015;26:67-74. [Crossref] [PubMed]
- Hsu LW, Goto S, Nakano T, et al. Prolonged survival by combined treatment with granulocyte colony-stimulating factor and dipeptidyl peptidase IV inhibitor in a rat small-for-size liver transplantation model. Hepatol Res 2015;45:804-13. [Crossref] [PubMed]
- Chen X, Murad M, Cui YY, et al. miRNA regulation of liver growth after 50% partial hepatectomy and small size grafts in rats. Transplantation 2011;91:293-9. [Crossref] [PubMed]
- Iguchi K, Hatano E, Yamanaka K, et al. Hepatoprotective effect by pretreatment with olprinone in a swine partial hepatectomy model. Liver Transpl 2014;20:838-49. [Crossref] [PubMed]
- Nakano T, Lai CY, Goto S, et al. Immunological and regenerative aspects of hepatic mast cells in liver allograft rejection and tolerance. PLoS One 2012;7:e37202. [Crossref] [PubMed]
- Wei W, Huang XH, Liang D, et al. A proteomic analysis of transplanted liver in a rat model of chronic rejection. Clin Res Hepatol Gastroenterol 2015;39:340-50. [Crossref] [PubMed]
- Xu M, Tan C, Hu J, et al. Expression of hemopexin in acute rejection of rat liver allograft identified by serum proteomic analysis. Shock 2014;42:65-74. [Crossref] [PubMed]
- Cheng J, Zhou L, Jiang JW, et al. Proteomic analysis of differentially expressed proteins in rat liver allografts developed acute rejection. Eur Surg Res 2010;44:43-51. [Crossref] [PubMed]
- Wu Y, Tao Y, Liang L, et al. Metabonomic profile of rats with acute liver rejection. OMICS 2009;13:81-91. [Crossref] [PubMed]
- Gehrau R, Bontha S, Mas V, et al. DNA methylation patterns and association with graft injury severity post liver transplantation. Am J Transplant 2016;16:378-9.
- Taubert R, Pischke S, Schlue J, et al. Enrichment of regulatory T cells in acutely rejected human liver allografts. Am J Transplant 2012;12:3425-36. [Crossref] [PubMed]
- Joshi D, Salehi S, Brereton H, et al. Distinct microRNA profiles are associated with the severity of hepatitis C virus recurrence and acute cellular rejection after liver transplantation. Liver Transpl 2013;19:383-94. [Crossref] [PubMed]
- Uesugi M, Kikuchi M, Shinke H, et al. Impact of cytochrome P450 3A5 polymorphism in graft livers on the frequency of acute cellular rejection in living-donor liver transplantation. Pharmacogenet Genomics 2014;24:356-66. [Crossref] [PubMed]
- Bonaccorsi-Riani E, Pennycuick A, Londoño MC, et al. Molecular Characterization of Acute Cellular Rejection Occurring During Intentional Immunosuppression Withdrawal in Liver Transplantation. Am J Transplant 2016;16:484-96. [Crossref] [PubMed]
- Ningappa M, Ashokkumar C, Higgs BW, et al. Enhanced B Cell Alloantigen Presentation and Its Epigenetic Dysregulation in Liver Transplant Rejection. Am J Transplant 2016;16:497-508. [Crossref] [PubMed]
- Yagi S, Nagai K, Kadaba P, et al. A novel organ preservation for small partial liver transplantations in rats: venous systemic oxygen persufflation with nitric oxide gas. Am J Transplant 2013;13:222-8. [Crossref] [PubMed]
- Zhou S, Palanisamy AP, McGillicuddy JW, et al. New method of stent-facilitated arterial reconstruction for orthotopic mouse liver transplantation. J Surg Res 2014;187:297-301. [Crossref] [PubMed]
- Dong J, Xia L, Shen H, et al. Growing a whole porcine liver organ ex situ for six hours without red blood cells or hemoglobin. Am J Transl Res 2016;8:2562-74. [PubMed]
- Orlando G, Baptista P, Birchall M, et al. Regenerative medicine as applied to solid organ transplantation: current status and future challenges. Transpl Int 2011;24:223-32. [Crossref] [PubMed]
- Hashmi SK, Baranov E, Gonzalez A, et al. Genomics of liver transplant injury and regeneration. Transplant Rev (Orlando) 2015;29:23-32. [Crossref] [PubMed]
Cite this article as: Nacif LS, Kim V, Galvão F, Ono SK, Pinheiro RS, Carrilho FJ, D’Albuquerque LC. Translational medical research and liver transplantation: systematic review. Transl Gastroenterol Hepatol 2018;3:91.