
Treatment of advanced hepatocellular carcinoma: immunotherapy from checkpoint blockade to potential of cellular treatment
Current strategies of hepatocellular carcinoma (HCC) treatments
HCC is the predominantly histological subtype of primary liver cancer, accounts for the fifth most common malignancy and the second leading cause of cancer-related death globally, in accordance with the Global Burden of Disease Study 2013 (1). Technical advances in imaging have facilitated the diagnosis of early-stage HCCs, with approximately 30–40% of patients are administered for radical treatments such as surgical resection, transplantation, and ablation. Barcelona clinic liver cancer (BCLC) staging classification has been introduced to manage therapeutic decisions (2), considering factors such as tumor burden, reserved hepatic function, treatment allocation and prognosis, allowing the early staged patients to achieve a median overall survival over 60 months and 5-year survival of 50–80% (3,4). Despite that, metastases and de novo carcinogenesis occur even upon curative therapeutics, and the efficacy of adjuvant chemo- or radiation-therapy are suboptimal (5), with substantial 5-year recurrence rate reaches up to 70% (6).
The prognosis of patients with advanced HCC presents a more challenging clinical scenario, with an overall survival of 11 months (7) and an overall 5-year survival rate less than 16% (8). Systemic chemotherapies have restricted therapeutic implications as only marginal clinical benefits of 10–20% response rates have been shown and weak tolerance for patients with accompanying liver cirrhosis. Patients at intermediate stage or advanced stage tend to adhere to palliative therapies including chemoembolization and sorafenib. Sorafenib, an oral multitargeted tyrosine kinase inhibitor, was the sole pharmacological treatment that has been prevailed with effectiveness for advanced HCC. It has been clinically adopted for patients with advanced HCC as first-line therapy for more than a decade. However modest overall survival benefits have been shown, with a suboptimal efficacy of 3 months prolonged overall survival (9) and no predictive biomarkers of responsiveness to sorafenib have been identified. Treatment options remain limited for advanced HCC. Given its poor prognosis, extensive research on the dissection of the molecular pathogenesis of HCC has led to the identification of more than 160 oncogenes (10); however, subsequent druggable targets have not been identified. Over the years, there have been many disappointments in phase III clinical trials on drug development for HCC. The failures in the past decade can be partially explained by the high heterogeneity in the molecular and biological behavior of HCC pathogenesis, lack of key biomarkers for stratification of patients, toxicity and resistance to the conventional chemotherapy or systemic agents.
New treatment modalities to prolong survival and minimize the risk of adverse responses for patients with advanced HCCs are not demonstrated until very recently. Regorafenib (angiogenesis inhibitor) and nivolumab (an immune checkpoint inhibitor) were approved as second-line HCC treatment for patients who failed prior sorafenib treatment, based on persuasive efficacy in the clinical trials (11,12). Other agents including lenvatinib, durvalumab and tremelimumab are being evaluated in first-line unresectable HCC as a single agent or in combination. In fact, cancer immunotherapy has been exemplified as the Science “Breakthrough of the Year” in 2013. In particular, immune checkpoints inhibitors have shown promising results in patients with hematological malignancies as well as solid tumors and approved for the following indications: Hodgkin’s lymphoma, advanced melanoma, non-small cell lung cancer, renal-cell carcinoma, head and neck cancer, merkel cell carcinoma, high microsatellite instability colorectal carcinoma and urothelial carcinoma (13). This class of agent has also been evaluated in patients with advanced HCC. The proven effectiveness of nivolumab in prolonging survival demonstrated by the recent clinical trial has been one of the most sensational developments (12).
Clinicopathological implications of T cell-mediated PD1/PD-L1 pathway in HCC pathogenesis
The liver is a unique organ with a very complicated and dynamic immune system that enables its role as first-line host defense against antigens and microbial products that originate from the intestine and systemic blood, without stimulating undesirable immune responses. Reportedly, the hepatic immune system can be deemed as “immunologically tolerogenic”, which, in conjunction, can be detrimental in the case of pathological conditions. This physiologic phenomenon can subvert immune response to inflammation and tumor development, wherein diseased liver conditions such as chronic viral inflammation with hepatitis B and C viruses, fibrosis and cirrhosis are prone to develop and eventually drive HCC emergence.
Since the recognition of immunoevasion as a cancer hallmark, sparking momentum in immunotherapeutics for HCC treatment has driven the exploitation of different modalities, for example, vaccination, cytokine therapy, and cell transfer immunotherapy. Despite the fact that liver is an immunologically privileged organ, these immune-based approaches failed to demonstrate clinical efficacy in advanced HCC. Multiple down-modulation mechanisms have been proposed to favor exacerbation of immunosuppressive signals, among which immune checkpoint pathways such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death 1 (PD-1), have shown to play vital roles in tumor progression. The PD-1 protein, termed “immune checkpoint”, represents a physiological regulatory machinery of the body to provide peripheral tolerance from autoimmune pathologies during chronic inflammations or infections. This immune protective pathway has been hijacked as an adaptive resistance mechanism for tumors to escape immune-mediated destruction, particularly the antigen-specific antitumor responses of T cells (14).
PD-1 (CD279) is an inhibitory molecule remarkably expressed on the surface of activated T cells (15). It is constitutively expressed on a wide variety of adaptive and innate immune cells including monocytes, B cells, NK cells, dendritic cells, regulatory T cell (Tregs), as well as the myeloid-derived suppressor cells (MDSCs) population. Programmed death-ligand (PD-L1) (B7-H1 and CD274), the primary ligand for PD-1, is expressed in antigen presenting cells (APCs). PD-L1 is frequently found to be upregulated across tumor types as an immunoevasion machinery (16), yet the pathways and factors that modulate its expression remain obscure. Growing findings have suggested that during tumorigenesis, expression of PD-L1 can be driven by various oncogenic events depends on histotypes and mutations. For example, in mutated phosphatase and tensin homolog (PTEN) tumors, loss of PTEN can lead to phosphatidylinositol-3 kinase (PI3K) activation of protein kinase B (Akt) pathway, which in turn enhances transcription of PD-L1 messenger RNA (17). Activation of STAT3 transcription factor and NF-κB can also facilitate PD-L1 mRNA transcription (18,19) (Figure 1) (20). Inflammatory responses of activated T cells under antigen presentation can also implicate in upregulation of PD-L1 expression through secretion of multiple pro-inflammatory molecules. Among them, the interferon-gamma (IFN-γ) dependent loop is reported as the most pivotal inducer. IFN-γ is produced by tumor-associated antigen (TAA)-specific T cells in the tumor microenvironment. Interferon-gamma receptors (INFGR) of the tumor cells then take part in the activation of Jak/Stat pathway, which exerts a permissive role on PD-L1 transcription through downstream interferon regulatory factor-1 (IRF-1) (18). Hence, not only immunoevasion, in which tumors evolve to dampen the host immune surveillance, it is now conceivable that the immunity can also “edit” the tumor cells to escape from immunosurveillance, termed “immunoediting”. PD-L2 is another cognate ligand of PD-1 which has a different expression pattern. It is predominantly expressed on antigen-presenting cells including macrophages, dendritic cells and mast cells, and much less prevalent across aggressive human cancers. The binding affinity of PD-1 with PD-L2 is much less than that of between PD-1 and PD-L1. Taken together, the PD-1/PD-L1 axis remains as the mainstay hallmark to evade antitumor responses.
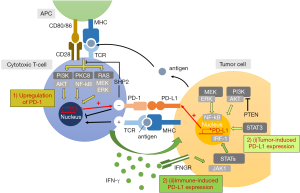
Extensive researches on the immune regulation of PD-1/PD-L1 inhibitory receptor and ligand pair mediated by T cells has emerged us a clearer perceptive on the distinct immunological mechanisms relevant for anergy of immune cells. PD-L1 is upregulated under chronic antigen exposure, and upon engagement with PD-1 in the tumor microenvironment, dephosphorylation at the downstream of T cell receptor (TCR) signaling is induced, resulting in the dysfunction of the cytotoxic T cells. Subsequently, inhibition of helper T cells can be developed under the secretion of immunosuppressive cytokines. Numerous studies have reported that engagement of PD-1 with PD-L1 can inhibit T cell activation, proliferation, and cytokine production, and ultimately results in exhausted effector T cells (21-23). Indeed, their expression has been extensively elucidated to prognosis adverse outcomes across cancers (24).
Patients with chronic inflamed liver disease have shown overexpression of PD-1 in the intrahepatic lymphocytes population while expression of its ligands, PD-L1 and PD-L2, were found on the Kupffer cells, liver sinusoidal endothelial cells and leukocytes (25,26), resulting to inhibition of cytotoxic and helper T lymphocytes (27). Studies have shown patients with hepatitis (HBV or HCV) or cirrhosis expressed with a significantly higher frequency of PD-1 in their peripheral and intrahepatic cytotoxic T cells (CTL) are prone to HCC development. The in vitro study further shown the CTLs have induced PD-L1 expression on hepatoma cells in an IFN-γ-dependent manner and PD-1/PD-L1 blockade can rescue the anergy of CTLs (28-30). Collectively, these studies provided hints that the PD-axis involves even at the early stage of pathogenesis, i.e., liver injury and inflammation, as HCC is a chronic inflammation-provoking disease. Upregulation of PD-1 has been observed in circulating and intratumor CTLs of HCC patients (30), whereas PD-L1 overexpression was seen in intratumor hepatoma cells and peritumoral stromal cells (16,30). Their overexpression is associated with HCC stages, poor prognosis, and postoperative recurrences (16,30-33).
Therapeutic significance of immune checkpoint inhibition
Current insights into the mechanism of action for PD-1 checkpoint have opened new perspectives to provoke tumor-specific immune responses. A novel class of immunotherapeutic agent is thus fostered by blockade of the PD-1 pathway utilizing monoclonal antibodies to inhibit either PD-1 on activated T cells or PD-L1 on tumor cells, as the PD-1/PD-L1 pathway is initiated by ligand-receptor interaction. These blocking antibodies against PD-1/PD-L1 are attempted to reduce suppressive signal and restore the activity of the effector T cells to mediate the tumor antigen-specific killing. Unprecedentedly, they have successful translated from the bench to the bedside over the last few years and proven to have the most widespread benefit as a single agent, with unequivocal signs of antitumor efficacy across a spectrum of tumor types including HCC.
HCC is typically non-immunogenic cancer with dysregulated immunotolerance, yet there is a paradox of the immunological role of its antigen-presenting system. Spontaneous T cell immune responses against TAAs have been reported previously in HCC patients, with correlation to improved prognosis (34,35). Further, the liver presents a unique immunologic milieu with APCs, such as resident dendritic cells, hepatocytes, hepatic stellate cells, Kupffer cells and more particularly liver sinusoidal endothelial cells, which are potential immunomodulating administrators under manipulation, as these immune cells have intrinsic innate immune functions supported by their pattern recognition receptors. Albeit the immunological role of each cell population to hepatocarcinogenesis has not been fully elucidated, it is believed that these cells may also define the immunosuppression of the PD pathway and can be targeted to shape the immune responses of the liver, for example, LSECs have been reported to express a high level of PD-L1 (27). Liver as a primary site for drug metabolism, hepatotoxicity burden is a prime concern as HCC patients are frequently associated with collateral liver dysfunction. Immune checkpoint inhibitors i.e., anti-PD-1/PD-L1 is a mild class of metabolic substrate compared to chemo drugs. Building on current understanding, it has also been suggested that anti-PD-1 is potentially expedient as the activation target can be tumor-infiltrating T cells as well as the population in the peripheral blood. Thus, a relatively low concentration of antibodies compared to conventional drugs is likely sufficient to initiate an immune response. Based on the physiological context of the liver, there is a strong rationale that HCC is amenable to immunotherapy. By contrast, the notion of treating HCC by small molecular drugs targeting oncogenic pathways lack credible bolster as druggable genes are rarely mutated in HCC.
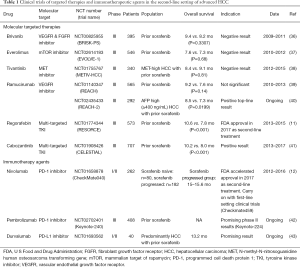
The broadened understanding of the mechanism underlying immune-inhibitory checkpoints, such as PD-1/PD-L1 and CTLA-4, has bestowed upon significant tumor regressions and antitumor activities of HCC patients reported in the preclinical and clinical trials of anti-PD-1/PD-L1 (Table 1). Nivolumab, a fully humanized IgG4 monoclonal antibody against PD-1 that is available in the market, has recently granted accelerated approval by the US Food and Drug Administration (FDA) for the treatment of advanced HCC patients refractory or intolerant to sorafenib treatment, based on the improved survival profiles and acquired high response rates in its phase I/II Checkmate-040 trial (NCT 01658878) (12). As detailed below, patients with advanced HCC who were not amenable to curative surgery or local treatment, irrespective of viral infection status, were enrolled in the trial. A durable objective response of 23% was displayed on the sorafenib-naïve patient group. For the sorafenib-experienced patients, promising therapeutic value with a median overall survival of 15–15.6 months and objective response rate of 16–19% were also reported. Notably, the toxicity profile of this drug was well tolerated compared to conventional chemotherapy, with mild and less frequent immune-mediated adverse reactions. The most common treatment-related adverse events reported were fatigue, pruritus, and rash. Although a comprehensive biomarker assessment was not demonstrated in this trial, an intriguing finding of no statistically differences towards the response rates between the PD-L1 expression-high- and PD-L1 expression-low-group was reported from the retrospective analysis, even with a low cut-off for membrane PD-L1 expression <1% of tumor cells measured (response rate of PD-L1-high vs. PD-L1-low: 26% vs. 19%). Collectively, it is therefore logical to conclude that nivolumab monotherapy is proven effective in advanced HCC patients regardless of viral etiology, sorafenib responsiveness, and PD-L1 expression. Expansion cohorts were further recruited to evaluate the safety and tolerability of the combinations of the checkpoints inhibitors (nivolumab + cabozantinib +/− ipilimumab). Subsequently, the implication of the potential benefit of early adoption of immunotherapy has led to the evaluation of nivolumab in first-line for advanced HCC in a global phase III randomized trial which is now well underway (Checkmate-459, NCT02576509) (44). Top-line results are expected in the second half of 2018.
Full table
To date, phase II study has reported a response rate of 18% (45) and the ongoing phase III study of pembrolizumab (anti-PD-1 antibody) versus placebo in the second-line setting has also gained encouraging interim results and expected to be eligible HCC treatment in the near future (Keynote-240, NCT02702401) (42). It has gained approval for the treatment of melanoma and non-small-cell lung cancer. While clinical trials of various immune checkpoints inhibitors are undertaken, design to target non-redundant pathways of the paramount immune checkpoints, for instance, a phase I/II clinical study that investigates durvalumab (anti-PD-L1 antibody) and tremelimumab (anti-CTLA-4 antibody) as monotherapy and in combination for unresectable HCC is initiated (NCT02519348). Other agents, for example, tremelimumab, durvalumab, avelumab, and atezolizumab are under evaluation for other solid tumors. Simultaneously, plentiful collaborations of the industry and academia are taking place on combination regimes that incorporate additional strategies, for example, immunotherapeutic agents, molecular targeted therapies or locoregional treatments, with anti-PD-1/PD-L1 as the backbone (44). Previously, convincing signs of positive immunomodulatory effects have been reported on radiofrequency ablation (RFA), cryoablation (CA) and transarterial chemoembolization (TACE), which are used routinely for HCC patients in the intermediate stage. Investigations advocated that these conventional treatments can induce a peripheral immune response or local inflammation, resulting in tumor antigens generation and sensitize the active response to immunotherapy (46,47). A pilot study by Duffy and colleagues have shown that tremelimumab in combination with ablation resulted in positive clinical activity for advanced HCC patients (48) (NCT01853618). The more-in-depth trials assessing combinations of conventional therapies with novel agents are vigorously commenced.
Despite the remarkable clinical success of anti-PD-1/PD-L1 immunotherapy, a substantial proportion of patients fail to respond. Currently, it is not clear whether the PD-1 or PD-L1 inhibitors have a higher impact, wherein the efficacy of the PD inhibitors depends on different factors, for example, genomic features including tumor mutation burdens and oncogenic pathways, tumor-microenvironment, systemic immunity status, microbiome, and metastases (49-51). Several biomarkers, including high levels of PD-L1 expression on tumor cells, infiltrating T cells, mutations, low levels of immunosuppressive elements, and EMT/stem-like features have been suggested to be associated with a positive response to PD pathway blockade, but none of them have a definitive predictive value (52).
Indeed, despite the fact that the tumoral PD-L1 expression has shown to be associated with likelihood of the response to the PD pathway blockade in multiple cancer types (53-56), controversial findings of responsiveness of PD-L1-deficiency tumors against PD-1 blockade, including the aforementioned results of Checkmate-040, have encouraged further investigations on the potential contribution of PD-L1 from host immune cells (57,58). Gathering evidence elucidated that PD-L1 on both tumor cells and host immune cells can contribute to immune suppression (59-64). Yet discrepancies to the predictive power of this intrinsic biomarker appeared across histotypes and settings have precluded the utilization of PD-L1 expression as a biomarker. These disparities can be partially explained by technical limitations existed in the detection of PD-L1 expression by immunohistochemistry (IHC), subjected to antibodies, cutoff values, clinical sampling methods—timing, method and site of tissue specimens etc. Particularly, poor reliability in immune cell scoring was reported by Tsao and colleagues in the Blueprint phase 2 project (65). Standardization of PD-L1 IHC assay is likely to thrive an improved outlook for patient selection. In addition, next-generation sequencing is potential to serve as an alternative to provide more robust prognosis.
Contrary to our known understandings of the theory, corresponding studies have also shown enhanced T cell responses in the PD-1 deficient tumor microenvironment under PD-L1 inhibitor treatment (21-23). Efforts are undertaken to fully exploit the cellular and biological mechanisms of PD-L1 with other proteins in regulating immunosuppression. In this regard, neither of the expression of PD-1 nor PD-L1 is required for patient selection currently. In essence, PD-1 and PD-L1 level can be rather dynamic during tumorigenesis and course of treatment. A recent study by Chen and colleagues noted that rather than pre-treatment biopsies, early on-treatment biopsies may reveal stronger predictive value to treatment outcome (66). Therefore enrich patient population with paired pre- and on-treatment tumor biopsies is likely to expand the indications for this approach. The induction of a diagnostic test of serum or tissue biomarkers is the ideal immunotherapeutic scenario, notwithstanding, perhaps a more rational strategy is a combinatory score compiling the contributions of various indicators. Attention must be given to the viral infection status in consideration of the biomarkers of HCC, as chronic hepatitis is a prime risk factor of HCC development.
NK cells as a potential effector to PD pathway
As PD-1 is expressed by a wide spectrum of immune cells, it is not fully delineated which cellular components and mechanisms contribute to what extent of the immunoregulation of PD pathway. Despite the remarkable efficiency of T cell-mediated PD pathway blockade immunotherapy, increasing proficient studies have insights on the immune checkpoints expression of natural killer (NK) cells and its functional consequences. To promote the translational stride, studies that connect the biology of NK cells to the clinical methodologies must be comprehensively explored. In the following, current knowledge on NK cells in HCC are summarized with further discussion on the role of NK effector cell to immunomodulating functions involved in the PD pathway and correlative preclinical results.
NK cells are a critical lymphoid cell population of the innate immune system with adaptive immunity features (67), commonly characterized as CD3-CD56+ lymphocytes. They can response against pathogens (68) and now more evidence pointing to their regulatory role in tumor development (69). Mature NK cells are equipped with an intricate repertoire of activating and inhibiting transmembrane receptors that are germline gene encoded. As precedence, they can respond to the recognition of tumor cells in a major histocompatibility complex (MHC)-antigen-unrestricted-manner, which is distinct from the T and B effector cells that carry highly antigen-specific receptors produced by somatic gene rearrangements (70).
Constitutively, the activating receptors of NK cells recognize stress-induced ligands which usually develop under tumorigenesis, upon binding, phosphorylation is induced which further activates the downstream kinases, promoting NK cell degranulation against tumor cells and inflammatory cytokine secretion that help recruit adaptive immune cells (“stress-induced self” hypothesis) (71). Its counterpart, inhibitory receptors, recognize the self MHC-I complex, that presumably function as a protective regulatory mechanism to protect NK cells attack self (72,73). Tumor cells that either MHC-deficiency or expressed allogeneic MHC molecules (irrespective to the antigen recognition), therefore, release the potent brake of the NK cells by dampening the inhibitory signal (“missing-self” hypothesis) (Figure 2). The integration between activating and inhibitory signals transmitted by these cell surface receptors of NK cells tightly controls the activation in a complex analog manner.
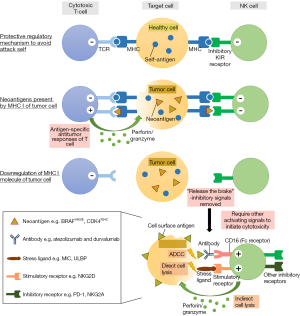
Once activated, NK cells not only can exert its natural, strong cytotoxic ability on the abnormal cells directly by delivering cytotoxic granules-perforin and granzymes, or by cell-cell contacts via the Fas ligand and tumor necrosis factor (TNF)-related apoptosis-induced ligand (74). It can also indirectly modulate the engagement of immune cells to the tumor site by secretion of inflammatory cytokines such as interferon-gamma (IFN-γ) and TNF-α and immuno-regulating cytokines including IL-3, GM-CSF, M-CSF (71,75,76). There are reciprocal interactions between NK cells and other immune cells from the innate and adaptive immune networks that help mount the immune responses (67,77). On top of that, NK cells are the major population that mediates antibody-dependent cell-mediated cytotoxicity (ADCC).
Tumor cells frequently lose the MHC-I complex and downregulate their tumor antigen as an adaptive evasion mechanism against antigen-specific T cells recognition. Decreased expression of the HLA molecules (human MHC class I protein) on the cell surface and defects in the antigen presentation machinery have been demonstrated on HCCs (78,79). As a result, the physiological significance of the T cell population may be less credible in the hepatic model. As a notable fact, high frequency of apoptotic T cells was reported in the intrahepatic lymphocytes population (80), yet the causal relationship between low levels of MHC molecule and apoptotic T cells accumulation have not been corroborated. In this context, NK cells may demonstrate an emerging role in hinder the immunoevasion in the hepatic immune system, as a complementary mechanism of action to T cells. Liver, unlike other organs, is predominantly harbored by the innate immune cells. The NK cells are selectively accumulated three times more in the liver compared to that of peripheral blood, constituting over one-third of the intrahepatic lymphocytes (81,82). The abundance of innate immune cells is likely explained by the defensive role of liver against intestinal antigens. Compelling evidence suggests that the hepatic NK cells are a group of unique NK subsets differ from the conventional NK cells of other lymphoid organs or peripheral blood, exhibit distinct phenotypic distribution and cytokine profiles, and more importantly stronger cytotoxicity (83,84). NK cell precursor population have also been reported to present in the liver, which implicates NK cells may have differentiated and proliferated in the liver in addition to bone marrow (82).
The role of NK cells in HCC oncogenesis was first demonstrated in a genetically engineered murine model, highlighting deregulated molecular mechanisms of NK cells take place during HCC onset and progression (85). Later on, there is substantial evidence linking the frequency of peripheral and intrahepatic NK cells to recurrence and survival in resectable HCC (86-89). These “liver-resident NK cells” therefore is now known to play a central role in the immune function of the liver and in the immune defenses against HCC (82,90). Such studies have laid the foundation for the clinical development of future NK cell-based immunotherapies for HCC treatment.
The eligibility and prospect of NK cells as candidate effector cells for immunotherapies are being rigorously pursued since its success employed in cytokine therapy and NK cell adoptive transfer for hematological malignancies. Particularly, cancer study of immune checkpoint therapies have been focused on antigen-specific T cells, but less is known about their functional consequences on NK cells. The lack of in-depth evaluation in the past is due to the uneven and low PD-1 expression of NK sub-populations. Herein the precise phenotype of NK cells is still controversial, as previously there have been proposed of CD56-negative cell identified in hepatitis C virus (HCV) (91) and other infections (75,92,93). Additionally, the expression of PD-1 on the surface of the NK subsets might be a dynamic matter that changes along tumor development and under treatment exposures. Difficulties in deciphering the expression alterations, infiltration and localization of each NK subsets in a longitudinal study have been a potential flag with current limitations of clinical sampling methods.
The positive role of NK cells is well established in the inflammation, infected and tumor model, while the immunosuppressive role of NK cells is not emerged until more recently. Accumulating evidence have pointing to the responsibility of NK cells to tumorigenesis via PD-1/PD-L1 immune evasion, including Hodgkin lymphoma (94), breast cancer (95), sarcoma (96) and in digestive cancers including esophageal, liver, colorectal, gastric and biliary cancer, as well as to the onset and progression of HCC (85). The initiation and expression of PD-1/PD-L1 on T cells provide a putative proxy for biochemical mechanisms on NK cells. In the recent study, it has shown that PD-1/PD-L1 blockade augmented phosphorylation of AKT in NK cell lines, thus the inhibitory effect on NK cells may somewhat analogous to the same downstream pathway of PD-axis as T cells through the mediation of PI3K/AKT signaling (97). PD-1 overexpression of peripheral NK subset from healthy individuals has provided a window to study the phenotypic and functional features of PD-1 to NK cells. The study has shown that the PD-1+ NK cells have presented with low proliferation and impaired antitumor activity which can be rescued by antibody to the PD pathway blockade (98). It has also been reported that a novel NK cell sub-population express this surface inhibitor receptors in some tumor types (99,100), and in chronic viral inflammation (101). Notably, PD-1 is found to be upregulated on tumor-infiltrating NK cells of HCC patients and correlated with shorter survival (97). More studies have dissected the role of PD-1/PD-L1 interactions on NK cells especially in compromising the function of other immune cells including DC activation and CD8+ T cell priming (102). These findings suggest the use of PD-1/PD-L1 blockade immune treatments aimed at restoration of NK cell activation as a possible therapeutic strategy to circumvent tumor escape and may provide a synergistic effect by manipulation of both T and NK cell-mediated immunosurveillance. Substantial preclinical and scatter clinical data are published to support PD-1 inhibitory not only applicable to enhance T cell-mediated immunotherapy, but also to restore the tumor-suppressive capacity of NK cells. The exhausted PD-1-enriched NK cells have shown to be restored with functionality by PD-1/PD-L1 blockade in different tumor setting including lymphoma (94), non-small cell lung carcinoma (103), pulmonary metastases (104), esophageal squamous cell carcinoma (97) and brain cancer (105). The implication of NK cell-mediated inhibitory effect of PD-axis is likely to complicate the picture of immune surveillance, but it is believed to have an additive effect on clinical outcome of the therapeutic PD-1 blockade.
Noteworthy, in multiple tumor types, an upregulation of tumor antigens that are ligands to NKG2D, an activating receptor of NK cells, was found in the cancer stem cell (CSC) population, including pancreatic cancers, breast cancers, and sarcomas (106-110). The CSCs are the cell population that capable of long-term self-renewing in the tumor nests in which the mechanisms of relapse are highly conserved. They are considered as a key regulator participation in drug resistance (111). Consequently, it is suggested that activated NK cells have a superior antitumor ability in preferentially target cancer cells with a CSC phenotype. Single-cell analysis performed on HCC has shown intratumor molecular heterogeneity of hepatic CSCs (112), which may account for the formidable failures of HCC treatment in the past decade. NK-based CSC therapies are potent to pave an entirely revolutionized treatment algorithm against and represent a feasible opportunity to attenuate primary or acquired resistance for immunotherapy in the future. This potential life-prolonging approach deserves further prolongation.
Conclusions
Here, we present a review of the immune response of HCC and the mechanism of action of underlying the PD-1/PD-L1 pathways, with a further summarization of ongoing relevant clinical trials to provide an outlook of future perspectives on immune checkpoint inhibitors in the field of HCC immunotherapy. We have emphasized on the emerging role of NK cells as a potential effector of the PD axis. With the theoretical advantages of NK cells in tumor cell recognition and antitumor ability, NK-mediated checkpoints inhibitions are postulated to conquer this intractable disease as complementarity to T cell. A better understanding of NK cell activation and manipulation in the tumor immune microenvironment of the hepatic system will advance the clinical development of NK-based therapy and management. Multimodal therapeutic strategies, i.e., immune checkpoint inhibitors with chemotherapies, locoregional therapies or cellular therapies, are likely the next generation of therapeutic management for HCC.
Acknowledgements
Funding: This work was supported in part by Health and Medical Research Fund (05160556), the Terry Fox Foundation and Hong Kong Research Grants Council (T12-401/13-R).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-71. [Crossref] [PubMed]
- Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol 2013;10:34-42. [Crossref] [PubMed]
- Akoad ME, Pomfret EA. Surgical resection and liver transplantation for hepatocellular carcinoma. Clin Liver Dis 2015;19:381-99. [Crossref] [PubMed]
- Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569-77. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200-7. [Crossref] [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 2015;47:505-11. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-82. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252. [Crossref] [PubMed]
- Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996;8:765-72. [Crossref] [PubMed]
- Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971-9. [Crossref] [PubMed]
- Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007;13:84-8. [Crossref] [PubMed]
- Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol 2014;193:3835-41. [Crossref] [PubMed]
- Chen J, Jiang CC, Jin L, et al. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 2016;27:409-16. [Crossref] [PubMed]
- Kythreotou A, Siddique A, Mauri FA, et al. PD-L1. J Clin Pathol 2018;71:189-94. [Crossref] [PubMed]
- Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010;116:1291-8. [Crossref] [PubMed]
- Yang J, Riella LV, Chock S, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol 2011;187:1113-9. [Crossref] [PubMed]
- Paterson AM, Brown KE, Keir ME, et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol 2011;187:1097-105. [Crossref] [PubMed]
- Sznol M, Chen L. Antagonist Antibodies to PD-1 and B7-H1 (PD-L1) in the Treatment of Advanced Human Cancer--response. Clin Cancer Res 2013;19:5542. [Crossref] [PubMed]
- Kassel R, Cruise MW, Iezzoni JC, et al. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology 2009;50:1625-37. [Crossref] [PubMed]
- Wang BJ, Bao JJ, Wang JZ, et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol 2011;17:3322-9. [Crossref] [PubMed]
- Diehl L, Schurich A, Grochtmann R, et al. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 2008;47:296-305. [Crossref] [PubMed]
- Nakamoto N, Cho H, Shaked A, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog 2009;5. [Crossref] [PubMed]
- Fisicaro P, Valdatta C, Massari M, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 2010;138:682-93, 93.e1-4.
- Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011;128:887-96. [Crossref] [PubMed]
- Gehring AJ, Ho ZZ, Tan AT, et al. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology 2009;137:682-90. [Crossref] [PubMed]
- Umemoto Y, Okano S, Matsumoto Y, et al. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol 2015;50:65-75. [Crossref] [PubMed]
- Yarchoan M, Xing D, Luan L, et al. Characterization of the Immune Microenvironment in Hepatocellular Carcinoma. Clin Cancer Res 2017;23:7333-9. [Crossref] [PubMed]
- Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. J Hepatol 2011;54:830-4. [Crossref] [PubMed]
- Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014;59:1415-26. [Crossref] [PubMed]
- Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509-16. [Crossref] [PubMed]
- Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67. [Crossref] [PubMed]
- Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol 2018;19:682-93. [Crossref] [PubMed]
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- Zhu AX, Galle PR, Kudo M, et al. A study of ramucirumab (LY3009806) versus placebo in patients with hepatocellular carcinoma and elevated baseline alpha-fetoprotein (REACH-2). J Clin Oncol 2018;36. [Crossref]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Finn RS, Chan SL, Zhu AX, et al. KEYNOTE-240: Randomized phase III study of pembrolizumab versus best supportive care for second-line advanced hepatocellular carcinoma. J Clin Oncol 2017;35. [Crossref]
- Wainberg ZA, Segal NH, Jaeger D, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J Clin Oncol 2017;35:4071. [Crossref]
- Sangro B, Park JW, Cruz CMD, et al. A randomized, multicenter, phase 3 study of nivolumab vs. sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J Clin Oncol 2016;34. [Crossref]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-52. [Crossref] [PubMed]
- Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Front Oncol 2015;4:385. [PubMed]
- Zerbini A, Pilli M, Penna A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res 2006;66:1139-46. [Crossref] [PubMed]
- Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:545-51. [Crossref] [PubMed]
- Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016;165:35-44. [Crossref] [PubMed]
- Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018;359:801-6. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Maleki Vareki S, Garrigos C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol 2017;116:116-24. [Crossref] [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8. [Crossref] [PubMed]
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. [Crossref] [PubMed]
- Tang F, Zheng P. Tumor cells versus host immune cells: whose PD-L1 contributes to PD-1/PD-L1 blockade mediated cancer immunotherapy? Cell Biosci 2018;8:34. [Crossref] [PubMed]
- Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 2018;128:805-15. [Crossref] [PubMed]
- Tang H, Liang Y, Anders RA, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest 2018;128:580-8. [Crossref] [PubMed]
- Juneja VR, McGuire KA, Manguso RT, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 2017;214:895-904. [Crossref] [PubMed]
- Kleinovink JW, van Hall T, Ossendorp F, et al. PD-L1 immune suppression in cancer: Tumor cells or host cells? Oncoimmunology 2017;6. [Crossref] [PubMed]
- Lau J, Cheung J, Navarro A, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun 2017;8:14572. [Crossref] [PubMed]
- Noguchi T, Ward JP, Gubin MM, et al. Temporally Distinct PD-L1 Expression by Tumor and Host Cells Contributes to Immune Escape. Cancer Immunol Res 2017;5:106-17. [Crossref] [PubMed]
- Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of Blueprint phase 2 project. J Thorac Oncol 2018;13:1302-11. [Crossref] [PubMed]
- Chen PL, Roh W, Reuben A, et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov 2016;6:827-37. [Crossref] [PubMed]
- Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011;331:44-9. [Crossref] [PubMed]
- Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol 2008;8:259-68. [Crossref] [PubMed]
- Moretta L, Bottino C, Pende D, et al. Surface NK receptors and their ligands on tumor cells. Semin Immunol 2006;18:151-8. [Crossref] [PubMed]
- Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol 2011;11:645-57. [Crossref] [PubMed]
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008;9:495-502. [Crossref] [PubMed]
- Lanier LL. NK cell recognition. Annu Rev Immunol 2005;23:225-74. [Crossref] [PubMed]
- Vivier E, Tomasello E, Baratin M, et al. Functions of natural killer cells. Nat Immunol 2008;9:503-10. [Crossref] [PubMed]
- Chua HL, Serov Y, Brahmi Z. Regulation of FasL expression in natural killer cells. Hum Immunol 2004;65:317-27. [Crossref] [PubMed]
- Bryceson YT, March ME, Ljunggren HG, et al. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006;107:159-66. [Crossref] [PubMed]
- Zwirner NW, Domaica CI. Cytokine regulation of natural killer cell effector functions. Biofactors 2010;36:274-88. [Crossref] [PubMed]
- Crome SQ, Lang PA, Lang KS, et al. Natural killer cells regulate diverse T cell responses. Trends Immunol 2013;34:342-9. [Crossref] [PubMed]
- Kurokohchi K, Carrington M, Mann DL, et al. Expression of HLA class I molecules and the transporter associated with antigen processing in hepatocellular carcinoma. Hepatology 1996;23:1181-8. [Crossref] [PubMed]
- Matsui M, Machida S, Itani-Yohda T, et al. Downregulation of the proteasome subunits, transporter, and antigen presentation in hepatocellular carcinoma, and their restoration by interferon-gamma. J Gastroenterol Hepatol 2002;17:897-907. [Crossref] [PubMed]
- Crispe IN, Dao T, Klugewitz K, et al. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev 2000;174:47-62. [Crossref] [PubMed]
- Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology 2008;47:729-36. [Crossref] [PubMed]
- Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology 2013;57:1654-62. [Crossref] [PubMed]
- Anfossi N, Andre P, Guia S, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006;25:331-42. [Crossref] [PubMed]
- Moroso V, Metselaar HJ, Mancham S, et al. Liver grafts contain a unique subset of natural killer cells that are transferred into the recipient after liver transplantation. Liver Transpl 2010;16:895-908. [Crossref] [PubMed]
- Coulouarn C, Factor VM, Conner EA, et al. Genomic modeling of tumor onset and progression in a mouse model of aggressive human liver cancer. Carcinogenesis 2011;32:1434-40. [Crossref] [PubMed]
- Cai L, Zhang Z, Zhou L, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008;129:428-37. [Crossref] [PubMed]
- Cariani E, Pilli M, Zerbini A, et al. HLA and killer immunoglobulin-like receptor genes as outcome predictors of hepatitis C virus-related hepatocellular carcinoma. Clin Cancer Res 2013;19:5465-73. [Crossref] [PubMed]
- Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012;61:427-38. [Crossref] [PubMed]
- Sun C, Sun HY, Xiao WH, et al. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin 2015;36:1191-9. [Crossref] [PubMed]
- Peng H, Wisse E, Tian Z. Liver natural killer cells: subsets and roles in liver immunity. Cell Mol Immunol 2016;13:328-36. [Crossref] [PubMed]
- Gonzalez VD, Falconer K, Bjorkstrom NK, et al. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J Immunol 2009;183:6612-8. [Crossref] [PubMed]
- Della Chiesa M, Falco M, Podesta M, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood 2012;119:399-410. [Crossref] [PubMed]
- Norris S, Coleman A, Kuri-Cervantes L, et al. PD-1 expression on natural killer cells and CD8(+) T cells during chronic HIV-1 infection. Viral Immunol 2012;25:329-32. [Crossref] [PubMed]
- Vari F, Arpon D, Keane C, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 2018;131:1809-19. [Crossref] [PubMed]
- Park IH, Yang HN, Lee KJ, et al. Tumor-derived IL-18 induces PD-1 expression on immunosuppressive NK cells in triple-negative breast cancer. Oncotarget 2017;8:32722-30. [PubMed]
- Beldi-Ferchiou A, Lambert M, Dogniaux S, et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 2016;7:72961-77. [Crossref] [PubMed]
- Liu Y, Cheng Y, Xu Y, et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 2017;36:6143-53. [Crossref] [PubMed]
- Pesce S, Greppi M, Tabellini G, et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol 2017;139:335-46.e3. [Crossref] [PubMed]
- MacFarlane AW 4th, Jillab M, Plimack ER, et al. PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection. Cancer Immunol Res 2014;2:320-31. [Crossref] [PubMed]
- Benson DM Jr, Bakan CE, Mishra A, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010;116:2286-94. [Crossref] [PubMed]
- Golden-Mason L, Klarquist J, Wahed AS, et al. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. J Immunol 2008;180:3637-41. [Crossref] [PubMed]
- Iraolagoitia XL, Spallanzani RG, Torres NI, et al. NK Cells Restrain Spontaneous Antitumor CD8+ T Cell Priming through PD-1/PD-L1 Interactions with Dendritic Cells. J Immunol 2016;197:953-61. [Crossref] [PubMed]
- Meraz IM, Majidi M, Cao X, et al. TUSC2 Immunogene Therapy Synergizes with Anti-PD-1 through Enhanced Proliferation and Infiltration of Natural Killer Cells in Syngeneic Kras-Mutant Mouse Lung Cancer Models. Cancer Immunol Res 2018;6:163-77. [Crossref] [PubMed]
- Ohs I, Ducimetiere L, Marinho J, et al. Restoration of Natural Killer Cell Antimetastatic Activity by IL12 and Checkpoint Blockade. Cancer Res 2017;77:7059-71. [Crossref] [PubMed]
- Huang BY, Zhan YP, Zong WJ, et al. The PD-1/B7-H1 pathway modulates the natural killer cells versus mouse glioma stem cells. PLoS One 2015;10. [Crossref] [PubMed]
- Grossenbacher SK, Canter RJ, Murphy WJ. Natural killer cell immunotherapy to target stem-like tumor cells. J Immunother Cancer 2016;4:19. [Crossref] [PubMed]
- Ames E, Canter RJ, Grossenbacher SK, et al. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J Immunol 2015;195:4010-9. [Crossref] [PubMed]
- Luna JI, Grossenbacher SK, Murphy WJ, et al. Targeting Cancer Stem Cells with Natural Killer Cell Immunotherapy. Expert Opin Biol Ther 2017;17:313-24. [Crossref] [PubMed]
- Ames E, Canter RJ, Grossenbacher SK, et al. Enhanced targeting of stem-like solid tumor cells with radiation and natural killer cells. Oncoimmunology 2015;4. [Crossref] [PubMed]
- Canter RJ, Grossenbacher SK, Ames E, et al. Immune targeting of cancer stem cells in gastrointestinal oncology. J Gastrointest Oncol 2016;7:S1-S10. [PubMed]
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013;501:328-37. [Crossref] [PubMed]
- Zheng H, Pomyen Y, Hernandez MO, et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology 2018;68:127-40. [Crossref] [PubMed]
Cite this article as: Siu EH, Chan AW, Chong CC, Chan SL, Lo KW, Cheung ST. Treatment of advanced hepatocellular carcinoma: immunotherapy from checkpoint blockade to potential of cellular treatment. Transl Gastroenterol Hepatol 2018;3:89.


