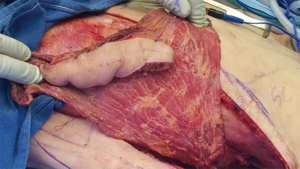
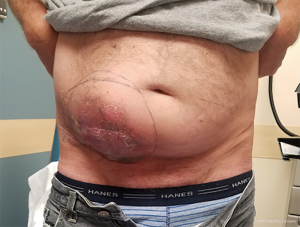
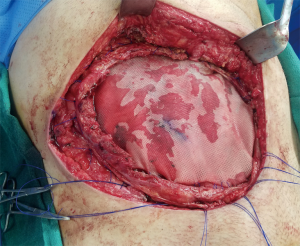
Incisions and reconstruction approaches for large sarcomas
Introduction
Intraabdominal, retroperitoneal, and abdominal wall sarcomas fall under the category of soft tissue sarcomas (STS), which are rare solid tumors of mesenchymal cell origin. Malignant mesenchymal neoplasms account for <1% of overall adult and 15% of pediatric malignant tumors of the human body but they carry a relatively high morbidity and a heterogeneity that can make treatment difficult (1-3). They tend to present at younger ages but do not have predominance amongst a gender or race. A total of 10–15% of sarcomas occur in the trunk and 15% in the retroperitoneum (4,5).
Prognosis is heavily defined by the grade of the sarcoma as measured by the mitotic activity, in addition to the histological subtype, but long-term survival is next determined by the completeness of the resection (4-9). Treatment is multifactorial but primarily centers on a R0 surgical resection that may require removal of adjacent viscera or portions of the abdominal wall. Local recurrence is directly correlated with the ability to achieve negative margins. Margins of at least 1 cm have been demonstrated especially in retroperitoneal sarcomas to increase the likelihood of local control (2,3,9). Margins that are found to be R0 on the final pathologic specimen but less than 1 cm may be recommended for re-excision and/or radiation therapy depending on the tumor location and grade.
This review focuses on surgical planning for intraabdominal, retroperitoneal, and abdominal wall STS’s and reconstruction options. Resection of these tumors and the resultant reconstruction may be conceptualized as a form of a ventral hernia repair or abdominal wall reconstruction, but frequently they are much more complex for a variety of factors. Their locations can demand non-traditional surgical incisions or full thickness loss of the abdominal wall that makes any repair more hernia-prone and pose the additional challenge of covering the defect with vascularized tissue. There may be an irradiated field if the tumor calls for neoadjuvant therapy, which completely changes the tissues quality and planes in the operative field. One may have to plan for possible post-reconstructive radiation and consider what repair would be the most resilient long-term following the adjuvant treatment. If the sarcoma involves intraabdominal or retroperitoneal viscera that must be resected, the contaminated field limits the prosthetic mesh options to reinforce repairs. Finally, as this is a cancer operation, there is a time factor that may not allow patients to be optimized pre-operatively as much as possible. Frequently prior to elective hernia repairs, patients are mandated to decrease their BMI’s, control their diabetes, start exercise and nutrition regimens, and have a complete cessation of all tobacco products. Surgeons do not have the luxury to delay these oncologic operations until these requirements are met, which will inherently place the patients at a higher risk for perioperative complications and long-term hernia occurrence.
Abdominal wall anatomy
A thorough understanding of abdominal wall anatomy is critical to preoperative planning and intraoperative decision making. Respecting the tissue layers and their vascular supplies is important in preventing hernias, maintaining skin viability, and preserving reconstructive options. For the purposes of this discussion, we will focus on the anterior abdominal wall. The anterior abdominal wall is often visualized as a hexagon. The hexagon is bordered superiorly by the costal margin with the apex at the xiphoid, laterally by the midaxillary lines, and inferiorly by the bilateral inguinal ligaments extending from the anterior superior iliac spine (ASIS) to pubic symphysis (10).
Musculature
The normal abdominal wall is made up of an inner muscular cylinder with a surrounding skin and soft tissue covering. The skin is the most superficial layer of the abdominal wall, and the dermis in this region is some of the thickest in the body. Deep to the skin, subcutaneous fat overlies the superficial fascia of the abdominal wall. Inferior to the umbilicus, the superficial fascia separates into two layers—Scarpa’s and Camper’s. Camper’s fascia is the more fatty, superficial layer and transitions caudally into the superficial fascia of the thighs. Scarpa’s fascia is the deeper, more fibrous layer that inferiorly joins the fascia lata of the thigh and Colle’s fascia in the perineum. A second layer of deeper fat is found between the superficial fascia and musculoaponeurotic layers (10,11).
The external oblique, internal oblique, and transversus abdominis muscles form the muscular layers of the lateral abdominal wall. The external oblique is the most superficial of the three, and its fibers are oriented with an inferomedial vector. The inferior oblique is in between external oblique and transversus. The internal oblique fibers are oriented superomedially, perpendicular to the external oblique. The transversus is the deepest muscle of the three and has horizontally oriented fibers. Laterally, parietal peritoneum is found immediately deep to the transversus abdominis (10,11).
Medially, the three lateral abdominal wall muscles form a fascial aponeurosis that centrally becomes the rectus sheath. The paired rectus abdominis muscles have vertically oriented fibers originating from the pubic symphysis and pubic crest and inserting on the xiphoid and 5th–7th costal cartilages. The aponeurotic layer begins lateral to the border of the rectus muscles, forms the rectus sheath surrounding the rectus muscle medially, and ultimately meets the contralateral aponeurosis in the midline to form the linea alba. The arcuate line is located at the level of the ASIS, about halfway between the umbilicus and pubic symphysis, and marks a major transition in the arrangement of the rectus sheath fascial layers. Superior to the arcuate line, the anterior rectus sheath is formed by external oblique aponeurosis with contributions from the internal oblique, and the posterior sheath is formed by the internal oblique and transversus abdominis. Below the arcuate line, the internal oblique and transversus aponeuroses only contribute to the anterior sheath; therefore, only a thin layer of relatively weak transversalis fascia is present posteriorly (10,11) (Figure 1).

Vascularity
Vascular supply to the anterior abdominal wall comes mainly from branches of the external iliac vessels, internal mammary vessels, lower intercostals, and lumbar vessels (12). This can be categorized into three main territories, called Huger’s zones (13). This is a very important concept in abdominal wall surgery, as previous abdominal incisions are indicators of which zones remain reliable. Zone I is central and extends from the xiphoid to ASIS, and the lateral edges of rectus muscles form its lateral borders. The deep inferior epigastric vessels from the external iliac and deep superior epigastric vessels from the internal mammary supply Zone I. Both give off several skin perforators before collateralizing near the level of the umbilicus. Zone II is located inferiorly on the abdomen bordered superiorly at the level of each ASIS and inferiorly by pubic symphysis and inguinal ligaments bilaterally. Zone II is supplied mainly by the superficial inferior epigastric and superficial circumflex femoral vessels with contributions from the external pudendal arteries and perforators from deep inferior epigastric artery. Zone III is made up of the lateral zones on each side of Zone I and superior to Zone II. Zone III is supplied by perforators from the lower six intercostal bundles and from lumbar arteries. There is rich collateralization of these source vessels as they feed the abdominal wall from different directions, and there is some overlap between each zone. The lateral abdominal wall musculature is supplied mainly by Zone III vessels while the rectus abdominis muscles are supplied by vessels of Zone I (10-13).
Innervation
The anterior abdominal wall receives most of its innervation from posteriorly and laterally from the 7th to 12th intercostal nerves and the first 3 lumbar nerves. The ventral rami at those levels provide innervation to abdominal wall muscles and sensation to the overlying soft tissue and skin. These nerves can be found travelling between the internal oblique muscles and transversus abdominis muscles and then piercing the posterior rectus sheath medial to the lateral borders of the rectus muscles. The ilioinguinal and iliohypogastric nerves are branches of the lumbar ventral nerves and travel inferiorly, branching in between the internal and external oblique muscles (10,11). They are both vulnerable to injury in that location and prone to forming neuromas. Knowledge of the abdominal wall innervation can help the surgeon prevent troublesome postoperative neuromas and can be a useful tool in controlling postoperative pain with locoregional nerve blocks.
Surgical incision planning
Intra-abdominal or retroperitoneal tumors
In the case of a purely intra-abdominal or retroperitoneal sarcoma, with no involvement of the abdominal wall or posterior trunk structures, it is recommended to proceed with a traditional midline laparotomy incision if feasible in order to maintain as much integrity of the abdominal wall as possible. That being said, an oncologically sound resection is paramount and may require multi-visceral en bloc resections depending on the primary tumor size and level of invasiveness. This may call for alternative incisions such as a chevron, subcostal, thoracoabdominal, or flank for the appropriate access. Retroperitoneal sarcomas are commonly approached with the patient in the lateral decubitus position not only to allow for the ideal surgical exposure but also to utilize gravity to separate the tumor from vital non-resectable structures (9).
Abdominal wall tumors
If the tumor is located within the abdominal wall, surgical incision is planned based on the anatomic location of the STS. Preservation of the superficial skin and soft tissue may be possible if the tumor is deep to Scarpa’s fascia but this may need to be determined at the time of surgery. Is should be considered though based on preoperative imaging when planning on skin incisions. Imaging should also be used to assess for intra-abdominal involvement of viscera that would require en bloc resection and may limit reconstruction options if contamination is present. The timing of definitive reconstruction must be optimized as much as possible to minimize the risk of reconstructive failure, wound infection, and hernia development. If there is any concern for positive margins on the resection specimen, wound contamination, or patient instability, the reconstruction should be done at a second stage (14). Alternatives for temporary closure include the ABthera™ (San Antonio, TX, USA) intraabdominal wound vac, traditional wound vac, Vicryl bridging mesh, or skin-only closure.
Partial thickness abdominal wall reconstruction
At times, partial abdominal wall resection may be appropriate if the tumor is small (<5 cm), low-grade, and located superficial to the abdominal fascia. In these cases, the soft tissue defects will generally be smaller even with the oncologically safe margins and are able to be closed primarily. If in the lower quadrants of the abdomen, a traditional panniculectomy with a separate resection of the skin of the contralateral side for symmetry and inferior advancement of the superiorly based adipocutaneous flap would result in an optimal aesthetic outcome. Additional options include skin grafting and local flaps, recruiting tissue from other areas of the abdominal wall and flanks through advancement, rotation, or transposition.
Full thickness abdominal wall reconstruction
If tumor size and location necessitate full thickness resection, the resultant defect dictates potential options for closure (5). Abdominal wall defects have previously been classified by Mathes et al. based upon location. Zone 1A involves an upper midline defect with extension across the midline. Zone 1B covers defects in the lower midline with extension across the midline. Zone 2 is an upper quadrant defect of the abdomen and Zone 3 is a lower quadrant defect of the abdomen (15). M.D. Anderson recently published a defect classification system to guide reconstructive surgeons following an oncologic resection of the abdominal wall. They divide the abdominal wall into 4 surface area types: type I is located within the 2 semilunar lines in the midcentral abdomen; type II is lateral to the semilunar lines; type III is cephalad to type I to the xiphoid process; and type IV is caudal to type I from the arcuate line to the pubic symphysis. There are additionally 3 depth subtypes: type A is skin and subcutaneous tissue only; type B is musculofascial abdominal wall only; and type C is skin, subcutaneous tissue, and any component of the musculofascial abdominal wall (16).
Primary closure techniques
Ideally, primary closure is performed if possible with or without component separation as utilized in traditional hernia repairs and abdominal wall reconstruction. The goals of abdominal wall reconstruction are to provide stable soft tissue coverage, restore fascial integrity, prevent hernia, protect abdominal viscera, and restore function if possible (16-18). These are generally reinforced with a prosthetic mesh to minimize risk of future hernia formation.
Component separation is a technique that is used to gain medial advancement of each side of the abdominal wall. Reported distances of 5 cm in the epigastrium, 10 cm at the waistline, and 3 cm in the suprapubic region may be achieved (18). In an anterior separation, external oblique aponeurosis is released 1.5 cm lateral to the linea semilunaris and dissection carried out in the avascular plane between the internal and external oblique muscles from the pubis to the costal margins (19) (Figure 2). This technique could be utilized bilaterally for central defects or only on the contralateral side of the resection to allow for primary closure.
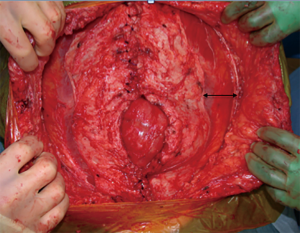
A posterior component release or a transversus abdominis muscle release (TAR) is another technique that is a modification of the Rives-Stoppa method. This allows for more fascial advancement than the Rives-Stoppa but still allows for mesh reinforcement inlay that is separate from the intraabdominal viscera. When performing the TAR, the posterior rectus sheath is incised about 0.5–1 cm from its edge at the level of the umbilicus. The retrorectus plane is developed laterally until 0.5 cm medial to the anterior-posterior rectus sheath junction and the posterior rectus sheath is incised. This reveals the underlying transversus abdominis muscle that is then divided along its entire medial edge and the plane between the transversalis fascia and the transversus abdominis muscle is developed. The posterior rectus sheath is reapproximated and then mesh is placed in the retromuscular space (20,21).
One may consider a staged repair with fascial tissue expansion using products like the Wittmann patch, but this would likely require a prolonged intensive care hospitalization, intubation, use of paralytics, and repeat procedures. This is most commonly used during damage control laparotomies when intra-abdominal edema precludes the ability to primarily close the abdomen without the risk of compartment syndrome but has also been utilized with hernia repairs when the loss of domain in unable to be overcome by component separation alone (22). This technique would then negate the need for a bridging mesh or tissue transfer, which in itself carries potential donor site morbidity or flap failure. It may be a reasonable alternative if a primary hernia repair is attempted with component separation but unable to fully close with a resultant small defect that would be able to be closed with one or two advancements of the patch.
Finally, if primary closure cannot be achieved, a bridging mesh may be considered in lieu of autologous tissue repair. Benefits include a relative expeditious closure and no donor site morbidity. Utilization of the techniques listed previously for skin and subcutaneous only resections can provide soft tissue coverage of the mesh. This would not be the preferred method though as bridging meshes have been shown to have higher rates of hernia recurrence, seroma, wound infection, and wound necrosis in comparison to hernia repairs performed with component separation (23,24). A bridging mesh repair would only be recommended in cases of large defects that were not amenable to primary repair with component separation and if the patient were not a candidate for autologous tissue transfer for other medical co-morbidities or lack of donor sites.
Vascularized tissue transfer techniques
Full thickness abdominal wall defects that are not amenable to repair via the previously described traditional hernia techniques may be covered with pedicled or free tissue transfer. There are several options based on the location and size of defect. Selection also depends on patient co-morbidities, ability to tolerate long reconstructive options, and donor site availability.
A pedicled omental flap may be used alone or in conjunction with a bridging prosthetic mesh to minimize the risks of mesh complications previously mentioned with this technique. If the omentum is uninvolved by the tumor, it is harvested on either the right or left gastroepiploic pedicle depending on the required arc of rotation for final inset. Reports of using the omentum alone to cover the abdominal wall defect and then either skin grafting or mobilizing local fasciocutaneous flaps for coverage have been made, but this leaves a hernia defect and inherent risk of evisceration (25). More commonly, the omentum is used along with a synthetic mesh and can be used as coverage alone or as a sandwich technique that splits the omentum and provides peritoneal lining in addition to external coverage of the prosthetic that can then be skin grafted (26,27). Omental flaps build upon a simple bridging mesh and preserve other reconstructive options if needed in the future.
The rectus abdominis flap muscle flap is a locoregional flap with a robust blood supply that allows it to be used anywhere on the anterior abdominal wall for full thickness defects (15,28). The deep inferior epigastric artery pedicle allows the flap to provide caudal coverage and the secondary pedicle is the superior epigastric artery that allows for cranial coverage (29). The musculofascial edges can be sutured to the edges of the resection bed. The major concern with rectus abdominis flaps is donor site morbidity and resultant hernia as it requires transferring abdominal wall strength from one location to another via both the rectus abdominis muscle and the anterior rectus sheath (30). The secondary defect may or may not be able to be closed primarily but it is generally recommended to reinforce either closure with a mesh to minimize ventral hernia or abdominal bulge (31,32). This addition of mesh does not affect abdominal wall strength in relation to flexion or rotation activities. Abdominal functional strength has been evaluated extensively in related to post-operative function following bilateral transverse rectus abdominis muscle (TRAM) flaps for breast reconstruction. This would likely correlate with oncologic resections on one side of the abdomen with sacrifice of the contralateral rectus muscle for rotational reconstruction. There is a demonstrable loss of strength in trunk flexion with bilateral TRAMs with some subjective reports of increased difficulty in activities in daily living, but this is mostly seen in activities like getting out of bed, which are directly based off of trunk flexion (33). Partial flap necrosis is an additional potential complication as the arc of rotation requires ligating one of the vascular pedicles, and the remaining one is not the dominant blood supply for the distal portion of the flap (32). Although there are benefits of the versatility of the flap, ease of harvest, and proximity to the resection, the morbidity of the donor site makes it less attractive than other locoregional or distant free tissue transfer options.
External oblique rotational flaps have been historically utilized for both abdominal and thoracic defects. The external oblique is a broad, thin muscle originating from the inferior borders of the lower eight ribs, run inferiorly and anteriorly, and then inserts on the xiphoid process, iliac crest, pubic tubercle, linea alba, inguinal ligament, and ASIS (34). Flap elevation may include a portion of the anterior rectus sheath, which provides a sturdy layer for inset and dissection is carried out laterally in the plane between the external and internal oblique muscles until the intercostal vessel perforators are visualized, as lateral as the posterior axillary line. This myofascial flap may also include the overlying soft tissue if the resection site is in need of additional skin coverage. Once raised, the muscle can be rotated clockwise or counterclockwise and it has been reported to cover defects as large as 300–500 cm2 (35). This could be utilized to cover either upper or lower defects on the depending on the area of resection and rotation direction of the flap but is best utilized for the upper two thirds of the abdomen with its limited arc of rotation (29). The donor site may be closed primarily or skin grafted and the fascia reinforced by fixating the internal oblique sheath to the linea alba to maintain its integrity.
The gracilis flap is a lower extremity muscular or myocutaneous flap that is very versatile and used for a wide variety of reconstructive procedures throughout the body. It can be used as both a free or pedicled flap based on the medial circumflex femoral artery, but most commonly described as a pedicled flap for lower abdominal and groin coverage given its origin at the ischiopubic ramus and insertion just distal to the medial condyle of the tibia (36,37). It was traditionally described as a muscle flap alone that could then be covered with a split-thickness skin graft, but may also include the overlying adipocutaneous soft tissue as well (38). In situ, the muscle belly is approximately 2 cm thick, 4 cm wide, and up to 30 cm in length, so its shape and size can be a limiting factor in its ability to cover larger defects. Wounds up to 240 cm2 have been reported for extremity coverage, but these are rectangular in shape and require scoring of the superficial epimysium in order to flatten the cylindrical shape (39). A reinforcing mesh for abdominal wall fascial defects should be utilized. Donor site morbidity is low with no significant functional deficits of the hip and knee and an aesthetically pleasing closure after harvest (40).
Rectus femoris flaps are best used as pedicled turnover or rotational flaps for lower abdominal defects. This can be taken as either a muscular or musculocutaneous flap and is supplied by the descending branch of the lateral circumflex femoral artery. It originates from the ASIS and the upper portion of the acetabulum with both heads joining together to insert into the patellar tendon (41). The distal portion of the flap can be divided at variable lengths proximal to the patellar tendon to preserve the stability of the knee, leaving in place the surrounding quadriceps muscles (vastus lateralis/intermedius/medialis). Strength and range of motion tests have been performed pre and post-operatively on patients who have undergone reconstructions utilizing the rectus femoris and have shown no significant decline in strength or donor site morbidity. These conclusions are made with the assumption that intra-operative technique of linking the vastus lateralis and vastus medialis muscles in the midline and a rigorous postoperative rehabilitation program maintain long-term function (42,43). It also should be used in conjunction with a reinforcing mesh for the abdominal wall fascial defect.
The latissimus dorsi (LD) flap is an additional myocutaneous reconstruction option that is commonly used for breast, head and neck, and trunk reconstruction. Abdominal wall use would require free tissue transfer based on the thoracodorsal primary pedicle (44). Once the surgical resection is complete, the deep inferior epigastric, superficial epigastric, or intraperitoneal gastroepiploic vessels may be selected as recipient based on tumor location and the appropriately sized muscular flap with or without a skin paddle is elevated (45) (Figures 3,4). A bridging mesh is sutured to the edges of the facial defect followed by the microvascular anastomosis and musculocutaneous inset. Donor site morbidity is low as it may be able to be closed primarily, although split thickness skin grafting may be required depending on body habitus and the size of the skin paddle. It has also been demonstrated that despite the muscle harvest which in situ contributes to shoulder adduction, extension, internal rotation, and scapular depression, long-term reported outcomes are positive with minimal to no functional deficits and overall satisfaction with donor site scars (46). This flap does have some negative aspects which has made is a less popular option than the next discussed thigh flaps. These include the need to change position intra-operatively as the harvest is performed in the lateral decubitus position and then the patient is turned supine for inset, as well as the fact that the LD does not have a fascial strength layer component so an additional layer mesh is always required to bridge the fascial defect.
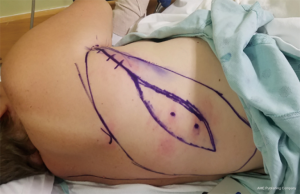
Tensor fascia lata (TFL) and anterolateral thigh (ALT) flaps have been proposed as the ideal flaps for large defects (>40 cm2) (29,47) (Figures 5,6). Both of these are vascularized from branches of the lateral femoral circumflex artery and may be used as a pedicled flap for lower abdominal defects or a free tissue transfer for the mid and upper abdomen individually or in conjunction if needed for bulk. Major benefits of these flaps are that they may be harvested with fascia, which can be sutured directly to the edges of the surrounding abdominal fascia, ability to fill a large area of potential dead space overlying the fascial reconstruction, and low donor site morbidity (15,18). The ability to incorporate autologous fascia into the repair reinstates as much integrity as possible to the abdominal wall and may negate the need to implant a prosthetic mesh. That being said, if only an ALT is selected or if there is any question of TFL integrity, an additional bridging mesh should be used for reinforcement. Utilization of either the ALT or TFL is dependent on the presence of adequate perforators (descending branch of the lateral femoral circumflex for the former, ascending branch for the latter) as well as recipient vessels if a free flap is to be performed (Figures 7,8). The most frequently used recipient vessels for abdominal wall reconstruction are the inferior epigastric, with intraperitoneal vessels like the gastroepiploic, femoral, internal thoracic and superior epigastric vessels used much less commonly (48). Donor sites may be closed primarily or skin grafted if needed and they result in no functional deficit (Figure 9).
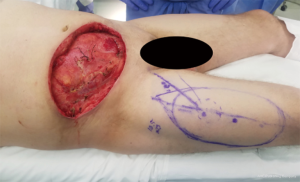
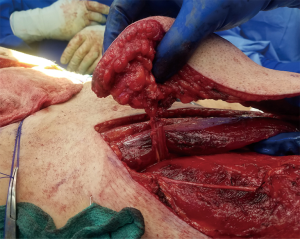
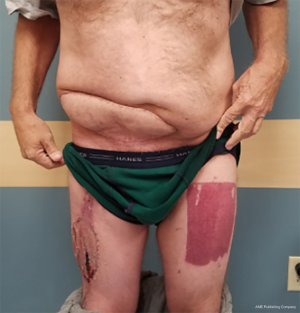
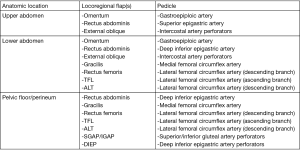
At times, pelvic reconstruction may be required either from direct extension from an intra-abdominal or retroperitoneal tumor or from a primary tumor stemming from the perineal soft tissues. In these cases, techniques commonly used to reconstruct defects secondary to urogynecologic or rectal cancers may be employed if a full thickness resection is required. Primary closure is difficult due to the limited mobility of the surrounding tissues unlike certain areas of the abdominal wall, but at times a fasciocutaneous V-Y advancement of the posterior thighs or the lateral gluteus maximus may be employed for perianal, sacral, or ischial lesions (49,50). The elevated tissue may also be de-epithelialized and buried subcutaneously to eradicate potential dead space and provide a multi-layered closure (Figures 10,11). Larger or deep space defects may be best treated with vascularized tissue transfers because not only can they cover large surface area defects, but they also offer more tissue bulk to obliterate dead space and provide a barrier adjacent to viscera to minimize risk of fistulization. Possible flaps include the aforementioned pedicled vertical or transverse rectus abdominis muscle (VRAM, TRAM), gracilis muscle, TFL, or rectus femoris muscle (51). Additionally, pedicled deep inferior epigastric perforator (DIEP) flaps are a muscle-sparing adipocutaneous alternatives that can offer a similar amount of volume in certain patient populations. Finally, gluteal perforator flaps are a regularly employed technique unique to pelvic or perineal reconstruction, which are based off of the superior or inferior gluteal artery perforators (SGAP or IGAP). These have evolved from the gluteus muscle flap which has significant donor site morbidity in ambulatory patients and requires a difficult dissection with a resultant short pedicle (52).

Conclusions
Intra-abdominal, retroperitoneal, and abdominal wall sarcomas are a relatively rare occurrence in surgical oncology, but can provide unique challenges surrounding both resection and reconstruction. Their invasive nature and ability to grow to impressive sizes in the retroperitoneum frequently call for sizeable incisions, visceral resections, and possible full thickness loss of a portion of the abdominal wall. Over time, several techniques have been developed surrounding large ventral hernia repairs and abdominal wall reconstructions with respect to better identifying blood supplies, component separation, and innovation with prosthetic materials that have been translated into oncologic abdominal wall reconstruction with appreciable success in immediate coverage as well as minimizing future hernia development. These methods combined with other commonly utilized procedures of vascularized tissue transfers (Figure 12) amongst plastic surgeons complement the armamentarium of reconstructive options for patients who may otherwise have a devastating outcome with a prohibitive resection. Now with the proper multi-disciplinary diagnosis, work-up, and operative planning amongst surgical and medical oncologists and reconstructive surgeons, these sarcomas have an increased likelihood of achieving an oncologically sound resection with a subsequent functional reconstruction.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Husain N, Verma N. Current concepts in pathology of soft tissue sarcoma. Indian J Surg Oncol 2011;2:302-8. [Crossref] [PubMed]
- Stojadinovic A, Hoos A, Karpoff HM, et al. Soft tissue tumors of the abdominal wall: analysis of disease patterns and treatment. Arch Surg 2001;136:70-9. [Crossref] [PubMed]
- von Mehren M, Randall RL, Benjamin RS, et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:536-63. [Crossref] [PubMed]
- Mullinax JE, Zager JS, Gonzalez RJ. Current diagnosis and management of retroperitoneal sarcoma. Cancer Control 2011;18:177-87. [Crossref] [PubMed]
- Shiu MH, Weinstein L, Hajdu SI, et al. Malignant soft-tissue tumors of the anterior abdominal wall. Am J Surg 1989;158:446-51. [Crossref] [PubMed]
- Koniaris LG, Sola JE. Prognostication for trunk and retroperitoneal sarcomas. Ann Surg 2010;252:201. [Crossref] [PubMed]
- Gutierrez JC, Perez EA, Moffat FL, et al. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg 2007;245:952-8. [Crossref] [PubMed]
- Perez EA, Gutierrez JC, Moffat FL, et al. Retroperitoenal and truncal sarcomas: prognosis depends upon type and not location. Ann Surg Oncol 2007;14:1114-22. [Crossref] [PubMed]
- Ferrario T, Karakousis CP. Retroperitoneal sarcomas: grade and survival. Arch Surg 2003;138:248-51. [Crossref] [PubMed]
- Chim H, Evans KK, Mardini S. Abdominal wall anatomy and vascular supply. In: Rosen MJ. editor. Atlas of Abdominal Wall Reconstruction, 2nd ed. Amsterdam: Elsevier, 2017:2-20.
- Clemens MW, Butler CE. Abdominal wall reconstruction. In: Neligan PC. editor. Plastic Surgery: Volume 4: Lower Extremity, Trunk, and Burns. 3rd ed. Philadelphia: Saunders, 2012:276-91.
- Boyd JB, Taylor GI, Corlett R. The vascular territories of the superior epigastric and the deep inferior epigastric systems. Plast Reconstr Surg 1984;73:1-16. [Crossref] [PubMed]
- Huger WE. The anatomic rational for abdominal lipectomy. Am Surg 1979;45:612-7. [PubMed]
- Vijaykumar DK, Vijayaraghavan S. Reconstruction of chest, abdominal walls and perineum. Indian J Plast Surg 2007;40:90-8.
- Mathes SJ, Steinwald PM, Foster RD, et al. Complex abdominal wall reconstruction: a comparison of flap and mesh closure. Ann Surg 2000;232:586-96. [Crossref] [PubMed]
- Mericli AF, Baumann DP, Butler CE. Reconstruction of the Abdominal Wall after Oncologic Resection: Defect Classification and Management Strategies. Plast Reconstr Surg 2018;142:187S-96S. [Crossref] [PubMed]
- Khansa I, Janis JE. Modern reconstructive techniques for abdominal wall defects after oncologic resection. J Surg Oncol 2015;111:587-98. [Crossref] [PubMed]
- Rubayi S, Chandrasekhar BS. Trunk, abdomen, and pressure sore reconstruction. Plast Reconstr Surg 2011;128:201e-15e. [Crossref] [PubMed]
- Heller L, Chike-Obi C, Xue AS. Abdominal wall reconstruction with mesh and components separation. Semin Plast Surg 2012;26:29-35. [Crossref] [PubMed]
- Novitsky YW, Elliott HL, Orenstein SB, et al. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg 2012;204:709-16. [Crossref] [PubMed]
- Gazzola R, Lombardo M, Rosati R, et al. Posterior Component Separation with Transversus Abdominis Release: Technique, Utility, and Outcomes in Complex Abdominal Wall Reconstruction. Plast Reconstr Surg 2016;138:562e-3e. [Crossref] [PubMed]
- Huang Q, Li J, Lau WY. Techniques for Abdominal Wall Closure after Damage Control Laparotomy: From Temporary Abdominal Closure to Early/Delayed Fascial Closure-A Review. Gastroenterol Res Pract 2016;2016. [Crossref] [PubMed]
- Blatnik J, Jin J, Rosen M. Abdominal hernia repair with bridging acellular dermal matrix--an expensive hernia sac. Am J Surg 2008;196:47-50. [Crossref] [PubMed]
- Holihan JL, Askenasy EP, Greenberg JA, et al. Component Separation vs. Bridged Repair for Large Ventral Hernias: A Multi-Institutional Risk-Adjusted Comparison, Systematic Review, and Meta-Analysis. Surg Infect (Larchmt) 2016;17:17-26. [Crossref] [PubMed]
- da Silva AL. Repair of the anterior abdominal wall with omental flap. ABCD Arq Bras Cir Dig 2011;24:246-8.
- El-Muttardi N, Lancaster K, Ng R, et al. The sandwich omental flap for abdominal wall defect reconstruction. Br J Plast Surg 2005;58:841-4. [Crossref] [PubMed]
- Manay P, Khajanchi M, Prajapati R, et al. Pedicled omental and split skin graft in the reconstruction of the anterior abdominal wall. Int J Surg Case Rep 2014;5:161-3. [Crossref] [PubMed]
- Yoon CS, Kim CG, Kim H, et al. Reconstruction of infected trunk wounds with pedicled rectus abdominis musculocutaneous flaps: a retrospective case series. J Wound Care 2018;27:S4-11. [Crossref] [PubMed]
- Sacks JM, Broyles JM, Baumann DP. Flap coverage of anterior abdominal wall defects. Semin Plast Surg 2012;26:36-9. [Crossref] [PubMed]
- Erni D, Harder YD. The dissection of the rectus abdominis myocutaneous flap with complete preservation of the anterior rectus sheath. Br J Plast Surg 2003;56:395-400. [Crossref] [PubMed]
- Chirappapha P, Trikunagonvong N, Prapruttam D, et al. Donor-Site Complications and Remnant of Rectus Abdominis Muscle Status after Transverse Rectus Abdominis Myocutaneous Flap Reconstruction. Plast Reconstr Surg Glob Open 2017;5. [Crossref] [PubMed]
- Jeong W, Lee S, Kim J. Meta-analysis of flap perfusion and donor site complications for breast reconstruction using pedicled versus free TRAM and DIEP flaps. Breast 2018;38:45-51. [Crossref] [PubMed]
- Dulin WA, Avila RA, Verheyden CN, et al. Evaluation of abdominal wall strength after TRAM flap surgery. Plast Reconstr Surg 2004;113:1662-5; discussion 1666-7.
- Fan C, Fede C, Gaudreault N, et al. Anatomical and functional relationships between the external abdominal oblique muscle and the posterior layer of the thoracolumbar fascia. Clin Anat 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Wang CM, Zhang R, Luo P, et al. Reconstruction of extensive thoracic wall defect using the external oblique myocutaneous flap: An analysis on 20 Chinese patients with locally advanced soft tissue sarcoma. J Surg Oncol 2018;117:130-6. [Crossref] [PubMed]
- Magden O, Tayfur V, Edizer M, et al. Anatomy of gracilis muscle flap. J Craniofac Surg 2010;21:1948-50. [Crossref] [PubMed]
- Mujadzic T, Gober CA, Nahabedian DB, et al. Innervated pedicled gracilis flap for dynamic abdominal wall reconstruction. Plast Reconstr Surg Glob Open 2018;6. [Crossref] [PubMed]
- Douglas SR, Longo WE, Narayan D. A novel technique for perineal hernia repair. BMJ Case Rep 2013;2013. [Crossref] [PubMed]
- Calotta NA, Pedreira R, Deune EG. The Gracilis Free Flap Is a Viable Option for Large Extremity Wounds. Ann Plast Surg 2018;81:322-6. [Crossref] [PubMed]
- Besset M, Penaud A, Quignon R, et al. Donor site morbidity after free gracilis muscle flap. Report of 32 cases]. Ann Chir Plast Esthet 2014;59:53-60. [Crossref] [PubMed]
- Landim FM, Tavares JM, Costa ML, et al. Complex abdominal wall reconstruction after radiation therapy: a full-thickness defect was repaired with a rectus femoris myofasciocutaneous flap. Am J Obstet Gynecol 2009;200:116.e1-3. [Crossref] [PubMed]
- Caulfield WH, Curtsinger L, Powell G, et al. Donor leg morbidity after pedicled rectus femoris muscle flap transfer for abdominal wall and pelvic reconstruction. Ann Plast Surg 1994;32:377-82. [Crossref] [PubMed]
- Gardetto A, Raschner C, Schoeller T, et al. Rectus femoris muscle flap donor-site morbidity. Br J Plast Surg 2005;58:175-82. [Crossref] [PubMed]
- Maxwell GP. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg 1980;65:686-92. [Crossref] [PubMed]
- Bodin F, Dissaux C, Romain B, et al. Complex abdominal wall defect reconstruction using a latissimus dorsi free flap with mesh after malignant tumor resection. Microsurgery 2017;37:38-43. [Crossref] [PubMed]
- Koh E, Watson DI, Dean NR. Quality of life and shoulder function after latissimus dorsi breast reconstruction. J Plast Reconstr Aesthet Surg 2018;71:1317-23. [Crossref] [PubMed]
- Srinivas JS, Panagatla P, Damalacheru MR. Reconstruction of Type II abdominal wall defects: Anterolateral thigh or tensor fascia lata myocutaneous flaps? Indian J Plast Surg 2018;51:33-9. [Crossref] [PubMed]
- Gurunluoglu R, Rosen MJ. Recipient vessels for microsurgical flaps to the abdomen: A systematic review. Microsurgery 2017;37:707-16. [Crossref] [PubMed]
- Terashi H, Shibata O, Yamamoto A, et al. V-Y advancement posterior thigh fasciocutaneous flaps for total anal canal and large perianal defects. Ann Plast Surg 1996;37:340. [Crossref] [PubMed]
- Liu X, Lu W, Zhang Y, et al. Application of gluteus maximus fasciocutaneous V-Y advancement flap combined with resection in sacrococcygeal pressure ulcers. Medicine (Baltimore) 2017;96. [Crossref] [PubMed]
- Brodbeck R, Horch RE, Arkudas A, et al. Plastic and reconstructive surgery in the treatment of oncological perineal and genital defects. Front Oncol 2015;5:212. [Crossref] [PubMed]
- Gagnon AR, Blondeel PN. Superior gluteal artery perforator flap. Semin Plast Surg 2006;20:79-88. [Crossref]
Cite this article as: Spera LJ, Danforth RM, Hadad I. Incisions and reconstruction approaches for large sarcomas. Transl Gastroenterol Hepatol 2018;3:86.