Minimally invasive esophagectomy for Barrett’s adenocarcinoma
Introduction
Barrett’s esophagus is a metaplastic substitution of the squamous lining of the esophagus with columnar epithelium and is the only known precursor of esophageal adenocarcinoma (EAC). The main barriers to optimal prevention of Barrett’s esophagus include poor control of gastroesophageal reflux, late identification of Barrett’s esophagus, and the lack of methodology for appropriate risk-stratification (1). A global assessment based on data from eight Western countries through the year 2009 showed a continuing rise of EAC incidence at stable rates (2). It has also been estimated that by the year 2030, 1 in 100 men in the Netherlands and in the United Kingdom will be diagnosed with EAC (3). Esophagectomy is the contemporary mainstay of treatment for patients with locally invasive EAC staged T1sm/N+ or higher.
Trans-thoracic esophagectomy, first described by Ivor Lewis in 1944, is universally considered the gold standard surgical therapy for EAC. Compared to the technique of trans-hiatal esophagectomy without thoracotomy, the Ivor Lewis operation allows for wide margins of mediastinal lymphadenectomy. Minimally invasive esophageal surgery was proposed in the 1990s with the purpose to decrease the still high rate of respiratory complications secondary to the thoracotomy approach. Multiple minimally invasive surgical techniques have been developed and are presently used in referral institutions around the world. The most common hybrid procedure consists of a modified 2-stage Ivor Lewis operation where laparoscopy is used instead of laparotomy for the preparation of the gastric conduit (4). The total minimally invasive esophagectomy can be performed using two approaches: the 3-stage thoraco-laparoscopic esophagectomy (modified McKeown operation) where thoracoscopy and laparoscopy are used instead of right thoracotomy and laparotomy, and the minimally invasive trans-hiatal esophagectomy (modified 2-stage trans-hiatal esophagectomy), where laparoscopy is used instead of laparotomy (5-7). Therefore, minimally invasive/maximally effective esophagectomy has become a new and still evolving surgical paradigm.
Surgical planning
Patients with EAC require an extensive staging before surgery. This includes CT scan and/or endoscopic ultrasonography, and, when appropriate, flexible bronchoscopy and PET scan to rule out locally advanced cancer or distant metastases. Functional, nutritional, and comorbidity status evaluation is of paramount importance before either considering the patient for upfront surgery or planning a multimodality treatment as indicated by an interdisciplinary oncology board (8,9). In preparation for surgery, patients should refrain from smoking and exercise daily by walking and using an incentive spirometer; preoperative enteral nutrition for at least 1 week should be recommended to individuals with long history of dysphagia, marked weight loss, and a frail phenotype.
The choice of the surgical strategy is mainly influenced by the extent of the disease and tumor-related characteristics. Upfront laparoscopic approach for gastric conduit preparation, as part of a hybrid or total minimally invasive Ivor Lewis operation, is possible in most patients presenting with EAC. Initial thoracoscopy may instead be considered in patients with extra-long Barrett’s esophagus and when pleural carcinosis is suspected, especially if a large hiatus hernia is present. The presence of spine abnormalities such as kyphoscoliosis and osteophytosis, may represent a relative contraindication to thoracoscopy because of the potential technical difficulties to access the esophagus or the close anatomical relationships with the aorta and the tracheobronchial tree (10).
Surgical techniques
Minimally invasive Ivor Lewis esophagectomy
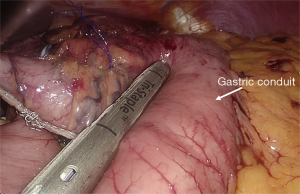
The hybrid operation includes a 2-stage approach, i.e., laparoscopy followed by right thoracotomy and an intrathoracic anastomosis. Laparoscopy is performed with the patient lying in a reverse Trendelenburg position. Stomach mobilization is accomplished by division the left gastric and short gastric vessels, and separation of the right gastroepiploic arcade from the greater omentum. A formal D2-type lymphadenectomy is performed. Finally, a 4-cm wide gastric conduit is constructed using a linear endostapler (Figure 1). Upon completion of laparoscopy, a left double-lumen tube or an endobronchial blocker under fiberoptic bronchoscopic guidance is placed; subsequently, the patient is turned to the left lateral position with a roll at the level of the tip of the scapula and a right postero-lateral thoracotomy is performed through the fifth intercostal space. After dividing the arch of the azygos vein, the esophagus and the overlying pleura is mobilized en bloc with the mediastinal nodes. The thoracic duct is ligated above the diaphragm. The mechanical esophago-gastric anastomosis is then performed at the apex of the right chest by means of a circular stapler.
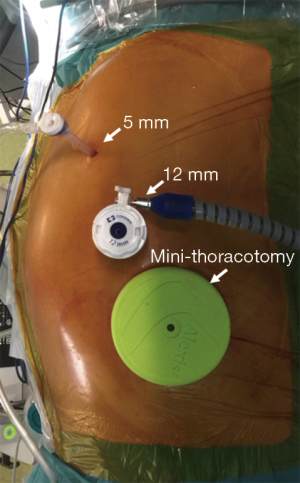
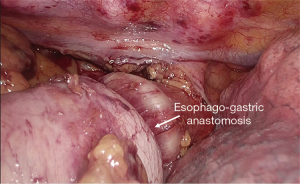
In the total minimally invasive Ivor Lewis esophagectomy, the laparoscopic stage of the operation is similar to that of the hybrid procedure. After completion of laparoscopy, the patient is turned into a prone or semi-prone position (Figure 2). Total esophageal mobilization and en-bloc mediastinal lymphadenectomy is performed. The esophagus is stapled at the level of the azygos vein and the anvil of a circular stapler (Orvil®, Medtronic) is inserted through the mouth and retrieved by the surgeon through a small esophagotomy close to the stapled end. Through a 5-cm mini-thoracotomy, the circular stapler is inserted with minimal dilatation of the intercostal space and advanced through a small gastrotomy to perform the intrathoracic esophago-gastric anastomosis (Figures 3,4).
Minimally invasive McKeown esophagectomy
This consists of a 3-stage esophagectomy with cervical anastomosis. Thoracoscopic esophageal mobilization in the prone position was first reported in 1992 (11). Other surgeons subsequently embraced the thoracoscopic technique with the patient lying in the left-lateral decubitus. Later on, the prone position was proposed again by Palanivelu in 2006 (12). Over time, it has been shown that the prone position has ergonomic and functional advantages compared to the lateral decubitus position (13). Esophageal mobilization and mediastinal lymphadenectomy are performed as previously described. Afterwards, the stomach is mobilized laparoscopically and the D2 celiac lymphadenectomy is performed. The previously transected esophagus is retrieved through the hiatus in the abdomen. A 4-cm wide gastric conduit is fashioned either extra-corporeally, through an upper midline minilaparotomy, or intra-corporeally, and then pulled under laparoscopic control up to the left neck incision through the posterior mediastinal tunnel. The cervical esophago-gastric anastomosis is performed using a linear endostapler.
Minimally invasive trans-hiatal esophagectomy
Two surgical teams may perform this operation to reduce operative time. The patient is supine on the operative table with the neck extended toward the left side. Gastric mobilization and celiac lymphadenectomy are performed through laparoscopy. Dissection of the esophagus and paraesophageal lymph nodes is performed up to the level of the inferior pulmonary vein. The cervical esophagus is then transected through a left cervicotomy and its distal stump is inverted and progressively retrieved in the abdomen under laparoscopic visualization. Construction of the gastric tube can be done extracorporeally through an upper midline minilaparotomy. Subsequently, the gastric tube is fixed to a chest tube and gently pushed up to the neck under laparoscopic visualization. Finally, a semi-mechanical side-to-side esophago-gastric anastomosis is performed in the neck.
Perioperative care
For selected patients undergoing minimally invasive esophagectomy, a standardized clinical pathway protocol may be followed. An epidural catheter is routinely placed, except in a few cases where contraindications exist. In such circumstances, a serratus anterior plane block can be performed (14). Routine preoperative antibiotic prophylaxis with Cefazolin is administered. An arterial line is always placed, but a central line is needed only in select patients. Intraoperatively, a warm air blanket is used to keep body temperature constant. Vasoconstrictors are avoided, and a maximum of 2 L of fluids are administered. Patients are generally extubated in the operating room. Nasogastric aspiration is maintained during the first 24–48 hours. Patients are allowed to walk and respiratory physiotherapy is initiated on postoperative day 1. On day 3, water sips and fruit jelly are allowed and the diet is gradually advanced. A gastrografin swallow study is performed on days 3–5 to check the anastomosis and gastric tube emptying. The following criteria should be met before hospital discharge: no evidence of infection, independent ambulation, no need for major analgesic drugs, well tolerated oral diet and no significant gastrointestinal discomfort.
Comment
Innovation and standardization in esophageal surgery has led to faster recovery, reduced postoperative mortality and improved oncological outcomes. A recent survey found a trend toward patient centralization and an increased adoption of minimally invasive esophagectomy. However, differences concerning the extent of lymphadenectomy and level of anastomosis still occur. Trans-hiatal esophagectomy is currently performed in about 15% of patients (15,16).
Choice of the surgical procedure
Minimally invasive esophagectomy can be performed with negligible blood loss, good pain control, decreased intensive care unit stay, and reduced incidence of respiratory complications. The early results of minimally invasive esophagectomy have proven at least equivalent to the open approach in meta-analyses (17,18), administrative national databases (19), and randomized clinical trials (20). In addition, minimally invasive esophagectomy has been associated with fast normalization of health-related quality of life (21). Further evidence of efficacy comes from a recent study providing the first “benchmark” data for clinically relevant endpoints in the 2-stage minimally invasive trans-thoracic esophagectomy (22).
Whether the decrease of major complication rate should mainly be attributed to the laparoscopic component of the hybrid Ivor Lewis operation remains unknown. Preliminary data from the Miro trial showed reduced major complication rates with similar oncological results and a trend toward better survival. In addition, when compared to open surgery, the hybrid Ivor Lewis approach was an independent factor protecting against respiratory complications (23,24). A more recent multicenter study also showed that laparoscopic gastric mobilization in the context of a hybrid esophagectomy significantly reduced postoperative 30- and 90-day mortality (25). Taken together, this data indicate that laparoscopy can alleviate the physical and immunological stress associated with one lung ventilation and lateral decubitus position. A total minimally invasive Ivor Lewis approach with thoracoscopic anastomosis would indeed be the ideal procedure, but its use is still limited due to the technical difficulties, the long learning curve, and low reproducibility of the anastomosis. Due to the increased incidence of EAC, all the efforts to embrace the total minimally invasive Ivor Lewis esophagectomy in the future are fully justified. It has been shown that 35–40 patients would be required for completing the learning curve (26), but still more evidence is needed to evaluate the efficacy of the various proposed techniques (27-29).
Adoption of the prone and semiprone position has indeed represented a significant advance in the performance of a 3-stage thoracoscopic esophagectomy by allowing 2-lung ventilation, and improved surgical ergonomics compared to the lateral decubitus (6,13). The TIME trial has proven that thoracoscopic prone esophagectomy is associated with a decreased incidence of in-hospital respiratory infections and hospital length of stay compared to the open approach (20). Furthermore, by avoiding the effect of the thoracotomy incision and by releasing the right chest and abdomen from compression during the prone position, improvement of oxygen delivery and of the ventilation-perfusion match, and decreased pulmonary shunt is expected. This may also contribute to decrease the incidence of respiratory and anastomotic complications. Last but not least, preserving bilateral lung ventilation can significantly reduce the ischemia-reperfusion injury and the oxidative stress (30,31).
In western countries, despite initial enthusiasms with the thoracoscopic approach, there has been a shift in favor of the 2-stage total minimally invasive Ivor Lewis approach. This may be have caused by an increased number of patients with EAC and by the concerns regarding the incidence of recurrent nerve injuries (32). Regarding the debate about the level of anastomosis, the semi-mechanical anastomosis in the neck can be considered safe as safe as the circular anastomosis at the top of the chest cavity, provided that both are performed in a standardized fashion (33).
With the development of new robotic platforms, the number of robotic esophagectomies may expand in the future due to the advantages of a stable three-dimensional image and a more precise surgical dissection. In addition, robotic surgery may enhance the ability to perform a safe manual anastomosis. A randomized clinical trial comparing open and robotic esophagectomy is ongoing (34). As new robotic systems will enter the market, the focus will be no longer on robotic arms and instruments but will shift more and more on integration with artificial intelligence to enhance surgeon capabilities. Last but not least, costs associated with the procedure are expected to decrease so the market of the robotic system will probably increase (35).
Minimally invasive esophagectomy has proven effective also in patients treated by neo-adjuvant chemoradiotherapy (20,36); however, a careful risk assessment is mandatory in individuals with large locally advanced tumors at initial presentation and in candidates to rescue surgery. In some circumstances, damage to the gastric fundus microcirculation by radiation may significantly increase the risk of anastomotic leakage in these patients (37). Evaluation of microperfusion with indocyanine green fluorescence angiography may help the surgeon to identify the most appropriate anastomotic site (Figure 5). From an oncological standpoint, the statistically significant increase in lymph node retrieval associated with minimally invasive esophagectomy was not associated with a survival benefit and no difference was demonstrated in 1-, 2-, 3- and 5-year survival rates (38). The updated 3-year results of the TIME trial showed a 40.2% disease-free survival in the minimally invasive group compared to 35.9% in the open group (39).

Postoperative quality of life
A very critical parameter in the management of EAC is postoperative quality of life. This is because the majority of 5-year survivors after esophagectomy show life-expectancy and quality of life levels comparable to the age-matched general population (40). The most common complaints after esophagectomy are gastroesophageal reflux, dysphagia, regurgitation, early satiety, delayed gastric emptying, dumping, weight loss, diarrhea, and fatigue. Longitudinal measurements of health-related quality of life (HRQOL) using the Short-form 36 and the EORTC questionnaires have the potential to guide shared decision-making by patients and physicians. Most patients functionally recover and regain quality of life during the first 2 years following Ivor Lewis esophagectomy (41). Maas et al. reported the 1-year outcomes of a randomized clinical trial comparing open and minimally invasive esophagectomy. Patients in the minimally invasive group had better quality of life scores compared to open esophagectomy in the global health, pain, and physical activity domains (42). An analysis comparing the open Ivor Lewis and the thoracoscopic McKeown esophagectomy found that the latter is associated with less pain and constipation (43). A meta-analysis of nine studies including 2,064 patients and comparing open and minimally invasive esophagectomy found better overall outcomes in patients undergoing minimally invasive esophagectomy at 4–6 weeks and at 3 months; better physical function persisted after 6 months (44).
Conclusions
The trans-thoracic Ivor Lewis esophagectomy is the preferred surgical approach in physiologically fit patients with Barrett’s adenocarcinoma. Minimally invasive techniques, including robotics, have contributed to augment surgical precision, reduce postoperative pain, and decrease the incidence of respiratory complications. The results of minimally invasive esophagectomy appear at least equivalent in terms of morbidity and mortality, nodal yield, completeness of resection, and early clinical and oncological results compared to open surgery. Quality of life appears improved by the laparoscopic/thoracoscopic approach. The role of minimally invasive surgery in the therapy of invasive EAC will continue to expand in synergy with enhanced recovery after surgery pathways. It is likely that the maximal gain in patient care will be reached once this multimodal process will be widely adopted and standardized to include prehospital, perioperative, intraoperative, and postoperative management components.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Inadomi J, Alastal H, Bonavina L, et al. Recent advances in Barrett's esophagus. Ann N Y Acad Sci 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Edgren G, Adami HO, Weiderpass E, et al. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2013;62:1406-14. Erratum in: Gut 2013;62:1820. Weiderpass Vainio, Elisabete [corrected to Weiderpass, Elisabete]. [Crossref] [PubMed]
- Arnold M, Laversanne M, Brown LM, et al. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol 2017;112:1247-55. [Crossref] [PubMed]
- Jagot P, Sauvanet A, Berthoux L, et al. Laparoscopic mobilization of the stomach for oesophageal replacement. Br J Surg 1996;83:540-2. [Crossref] [PubMed]
- Bonavina L, Bona D, Binyom PR, et al. A laparoscopy-assisted surgical approach to esophageal carcinoma. J Surg Res 2004;117:52-7. [Crossref] [PubMed]
- Bonavina L, Scolari F, Aiolfi A, et al. Early outcome of thoracoscopic and hybrid esophagectomy: propensity-matched comparative analysis. Surgery 2016;159:1073-81. [Crossref] [PubMed]
- Bonavina L, Asti E, Sironi A, et al. Hybrid and total minimally invasive esophagectomy: how I do it. J Thorac Dis 2017;9:S761-72. [Crossref] [PubMed]
- Basta YL, Bolle S, Fockens P, et al. The Value of Multidisciplinary Team Meetings for Patients with Gastrointestinal Malignancies: A Systematic Review. Ann Surg Oncol 2017;24:2669-78. [Crossref] [PubMed]
- Bernardi D, Asti E, Aiolfi A, et al. Outcome of Trimodal Therapy in Elderly Patients with Esophageal Cancer: Prognostic Value of the Charlson Comorbidity Index. Anticancer Res 2018;38:1815-20. [PubMed]
- Okamura A, Watanabe M, Mine S, et al. Factors influencing difficulty of the thoracic procedure in minimally invasive esophagectomy. Surg Endosc 2016;30:4279-85. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position. Experience of 130 patients. J Am Coll Surg 2006;203:7-16. [Crossref] [PubMed]
- Fabian T, Martin J, Katigbak M, et al. Thoracoscopic esophageal mobilization during minimally invasive esophagectomy: a head-to-head comparison of prone versus decubitus positions. Surg Endosc 2008;22:2485-91. [Crossref] [PubMed]
- Barbera C, Milito P, Punturieri M, et al. Serratus anterior plane block for hybrid transthoracic esophagectomy: a pilot study. J Pain Res 2017;10:73-7. [Crossref] [PubMed]
- Haverkamp L, Seesing MFJ, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Parry K, Ruurda JP, van der Sluis PC, et al. Current status of laparoscopic transhiatal esophagectomy for esophageal cancer patients: a systematic review of the literature. Dis Esophagus 2017;30:1-7. [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: metaanalysis of outcomes. Dig Dis Sci 2010;55:3031-40. [Crossref] [PubMed]
- Mamidanna R, Bottle A, Aylin P, et al. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg 2012;255:197-203. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Parameswaran R, Blazeby JM, Hughes R, et al. Health-related quality of life after minimally invasive oesophagectomy. Br J Surg 2010;97:525-31. [Crossref] [PubMed]
- Schmidt HM, Gisbertz SS, Moons J, et al. Defining Benchmarks for Transthoracic Esophagectomy: A Multicenter Analysis of Total Minimally Invasive Esophagectomy in Low Risk Patients. Ann Surg 2017;266:814-21. [Crossref] [PubMed]
- Briez N, Piessen G, Bonnetain F, et al. Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial – the MIRO trial. BMC Cancer 2011;11:310. [Crossref] [PubMed]
- Briez N, Piessen G, Torres F, et al. Effects of hybrid minimally invasive oesophagectomy on major postoperative pulmonary complications. Br J Surg 2012;99:1547-53. [Crossref] [PubMed]
- Messager M, Pasquer A, Duhamel A, et al. Laparoscopic gastric mobilization reduces postoperative mortality after esophageal cancer surgery: a French nationwide study. Ann Surg 2015;262:817-22. [Crossref] [PubMed]
- Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg 2014;218:1130-40. [Crossref] [PubMed]
- Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc 2012;26:1795-802. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Nieuwenhuijzen G, et al. Techniques and short-term outcomes for total minimally invasive Ivor Lewis esophageal resection in distal esophageal and gastroesophageal cancers: pooled data from six European centers. Surg Endosc 2017;31:119-26. [Crossref] [PubMed]
- Stenstra MHBC, van Workum F, van den Wildenberg FJH, et al. Evolution of the surgical technique of minimally invasive Ivor-Lewis esophagectomy: description according to the IDEAL framework. Dis Esophagus 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Bonavina L, Laface L, Abate E, et al. Comparison of ventilation and cardiovascular parameters between prone thoracoscopic and Ivor Lewis esophagectomy. Updates Surg 2012;64:81-5. [Crossref] [PubMed]
- Cheng YJ, Chan KC, Chien CT, et al. Oxidative stress during 1-lung ventilation. J Thorac Cardiovasc Surg 2006;132:513-8. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy. Review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction. A prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [Crossref] [PubMed]
- Gisbertz SS, Hagens ERC, Ruurda JP, et al. The evolution of surgical approach for esophageal cancer. Ann N Y Acad Sci 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Warner S, Chang YH, Paripati H, et al. Outcomes of minimally invasive esophagectomy in esophageal cancer after neoadjuvant chemoradiotherapy. Ann Thorac Surg 2014;97:439-45. [Crossref] [PubMed]
- Goense L, van Rossum PSN, Ruurda JP, et al. Radiation to the gastric fundus increases the risk of anastomotic leakage after esophagectomy. Ann Thorac Surg 2016;102:1798-804. [Crossref] [PubMed]
- Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg 2012;147:768-76. [Crossref] [PubMed]
- Straatman J, van der Vielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Derogar M, Lagergren P. Health-related quality of life among 5-year survivors of esophageal cancer surgery: a prospective, population-based study. J Clin Oncol 2012;30:413-8. [Crossref] [PubMed]
- Gutschow CA, Holscher AH, Leers J, et al. Health-related quality of life after Ivor Lewis esophagectomy. Langenbecks Arch Surg 2013;398:231-7. [Crossref] [PubMed]
- Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of Life and Late Complications After Minimally Invasive Compared to Open Esophagectomy: Results of a Randomized Trial. World J Surg 2015;39:1986-93. [Crossref] [PubMed]
- Barbour AP, Mc Cormack OM, Baker PJ, et al. Long-term health-related quality of life following esophagectomy. A nonrandomized comparison of thoracoscopically assisted and open surgery. Ann Surg 2017;265:1158-65. [Crossref] [PubMed]
- Kauppila JH, Xie S, Johar A, et al. Meta-analysis of health-related quality of life after minimally invasive versus open oesophagectomy for oesophageal cancer. Br J Surg 2017;104:1131-40. [Crossref] [PubMed]
Cite this article as: Asti E, Bernardi D, Sozzi M, Bonavina L. Minimally invasive esophagectomy for Barrett’s adenocarcinoma. Transl Gastroenterol Hepatol 2018;3:77.