Risk factors for colon location of cancer
World-wide, humans developed 1,096,601 colon and 704,376 rectal cancers during 2018, accounting for 10% of all new cases of cancer (1). Additionally, 551,269 people died of colon cancer and another 310,394 people died of rectal cancer, accounting for 9% of total cancer deaths (1). The disease is most prevalent in Western countries (1). In the US from 2009 to 2013, the distribution of cancers to the lower gastrointestinal tract was 41% in the proximal colon (proximal and including the splenic flexure), 22% in the distal colon (descending and sigmoid), 28% in the rectum, and 8% other sites (2). This anatomical site distribution slightly varied by gender, with females having 46%, 21%, 25%, and 8% for proximal, distal, rectum and other, respectively, and males having a 37%, 24%, 32%, and 7% distribution (2). There are a number of hypotheses that attempt to explain the varied distribution including why females develop more proximal cancers than men (3). These include the different embryological origins of the proximal and distal colons, variations in the concentration of bile salts and other compounds, level of oxygenation, and the microbial environment in different locations within the colon (3). Women overall live longer than men, and there is a distal-to-proximal shift of colon cancer as we age (4). The influence of sex hormones is also implicated (3). There are several morphologic and genetic observations between proximal and distal colon cancers that highlight the differences in colon location (5-7). Morphologically, flat sessile serrated adenomas and cancers are more commonly found in the proximal colon, and are difficult to observe without enhanced colonoscopic techniques, whereas polypoid adenomas and cancers are more common in the distal colon (3,5-7). Genetically, hypermethylation of the DNA mismatch repair gene MLH1 causing microsatellite instability is much more likely in proximal cancers, and tumors are diploid and generally lack TP53 mutations. Additionally, these sporadic microsatellite unstable cancers are also associated with the V600E mutation of BRAF (6). Chromosomal unstable tumors are more common in the distal colon, are aneuploid, and are driven by biallelic inactivation of APC and TP53 genes (6).
There are several implications regarding the approach to patient treatment based on the location of colorectal cancer (CRC). First, adjuvant and neoadjuvant therapy differ based on colon site, with rectal cancers treated at earlier stages (stage II) with a combination of chemotherapy and radiation therapy, as compared to colon cancers treated at stage III and do not get treated with radiation (3,8). Second, patients with microsatellite unstable CRC, who show improved prognosis compared to the matched-staged microsatellite stable cancer patient, do not respond favorably to 5-fluorouracil, the cornerstone of adjuvant therapy for CRC (9,10). Furthermore, microsatellite unstable cancer patients can benefit from immune checkpoint inhibitor therapy, which can further increase survival (11,12). Finally, there are approaches in using genetic or other biomarkers to personalize therapy for patients with CRCs. For instance, patients whose tumors contain KRAS mutation would not benefit from use of EGFR inhibitors, and patients with a PIK3CA mutation in their cancers may benefit from aspirin for secondary prevention (6).
Understanding the risk for CRC is just as important, as it has societal and policy implications regarding prevention beyond screening (7,13,14). CRC is a multifactorial disease where germline and somatic genetics, epigenetics and diet, lifestyle and the environment interplay (7,14). Risk factors that are not modifiable include age, family history, and race (7). These particular risk factors imply that the genetic makeup of an individual influences risk, and there is strong evidence for this (7,15). In particular, young age and/or family history of CRC may signify an inhertiable form of CRC, as 1 in 5 individuals with CRC less than 50 years of age demonstrate a germline mutation (MSH2, MLH1, MSH6, PMS2, APC, MUTYH, SMAD4, BRCA1, TP53, CHEK2) (16). However, about half of these young patients do not have family histories of cancer. Additionally, there is increasing evidence of young patients with CRC without identifiable germline mutations, particularly of the rectum and distal colon, that is an enigma currently (17). In the US, there are clear disparities for African Americans and CRC risk (7,14). Modifiable risk factors, meaning intervention should reduce risk, include dietary changes (e.g., high fiber, low red meat, low caloric) that likely influence the gut microbiome and obesity, physical activity, tobacco usage, menopausal hormone therapy, as well as socioeconomic and education status and screening utilization rates (7,18). These largely lifestyle risks are sometimes hard to change at the individual level, and generally require long-term utilization (e.g., dietary changes, cessation of tobacco, physical activity). Influencing populations at very young ages may have a larger societal effect, along with those who are motivated to live a healthy lifestyle. An example of this is anti-tobacco advertising, which has been in part responsible for the lower percentages of smoking in the US (19).
While there is consistency and epidemiological data showing the association of risk factors with the development or recurrence of CRC overall, there has not been any consistent data on the relevance of these risk factors with the specific colon location of CRC. In a recent issue of Clinical Gastroenterology and Hepatology, Murphy et al. published the largest cohort to study the relationship of CRC location in the colon and lifestyle factor association (20). Using the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort of 521,448 participants, the authors assessed 14 CRC risk factors that were measured at recruitment, and calculated hazard ratios using Cox proportional hazard models and heterogeneity Wald tests to determine relationships with anatomical site. Participants in EPIC were from 10 European countries (Denmark, France, Germany, Greece, Italy, Netherlands, Norway, Spain, Sweden and the UK) and the 14 risk factors measured were: (I) alcohol consumption; (II) non-steroidal anti-inflammatory drug (NSAID) use; (III) physical activity; (IV) presence of diabetes; (V) smoking status; (VI) body mass index (BMI); (VII) height; (VIII) waist circumference; (IX) waist-to-hip ratio; (X) age at menarche (females only); (XI) age at menopause (females only); (XII) oral contraceptive; (XIII) menopausal hormonal therapy; and (XIV) its duration of use (females only). Cancer incidence was determined through linkage with regional cancer registries and/or including use of health insurance records, pathology registries, and active follow-up. Exit time for patients in the study included, in the following order, CRC diagnosis, death, or the last date for follow-up. Average follow-up was 14.9 years. There were 6,291 CRC cases in 2,718 males and 3,573 females. Of these, 1,877 were proximal, 1,743 were distal, and 2,094 were rectal cancers (20).
Similar to prior studies, the authors identified alcohol consumption [hazard ratio (HR) 1.05; 95% confidence interval (CI): 1.03–1.07], prevalent diabetes (HR 1.28; 95% CI: 1.12–1.47), smoking (HR 1.19; 95% CI: 1.12–1.27), and higher anthropometric measurements (e.g., male BMI HR 1.22; 95% CI: 1.16–1.29) associated with a greater risk for CRC overall, and NSAID use (HR 0.85; 95% CI: 0.74–0.99), menopausal hormonal therapy (HR 0.90; 95% CI: 0.83–0.97), and active physical activity (HR 0.90; 95% CI: 0.82–0.98) associated with a lower risk for CRC overall (Figure 1). Regarding anatomical location in the colon, for physical activity, the inverse risk for CRC was most prevalent in the proximal colon (HR 0.74; 95% CI: 0.63–0.87) followed by the distal colon (HR 0.89; 95% CI: 0.76–1.05) and rectum (HR 1.06; 95% CI: 0.91–1.23), with both the distal and rectum locations not reaching statistical significance. For smoking, former smokers showed the highest risk in the distal colon (HR 1.27; 95% CI: 1.13–1.43), followed by rectum (HR 1.20; 95% CI: 1.09–1.33) and proximal colon (HR 1.12; 95% CI: 1.00–1.25). With alcohol consumption, there was no heterogeneity across sites meaning no relative difference in risk; however, the authors found positive association with distal (HR 1.07; 95% CI: 1.02–1.10) and rectal cancer locations (HR 1.07; 95% CI: 1.03–1.11). Similarly, there was no heterogeneity for relationships between diabetes and NSAID use across anatomical sites, meaning no relative difference for the risk in the proximal colon, distal colon, or rectum. For males but not females, BMI was associated with proximal (HR 1.31; 95% CI: 1.18–1.47) and distal (HR 1.32; 95% CI: 1.20–1.45) cancers more strongly than rectal cancers (HR 1.10; 95% CI: 1.01–1.20), trends similar for male waist circumference. Height for both males and females were associated with proximal (male HR 1.32; 95% CI: 1.17–1.48; female HR 1.30; 95% CI: 1.17–1.43) and distal (male HR 1.20; 95% CI: 1.07–1.34; female HR 1.11; 95% CI: 0.99–1.25) CRCs, but not rectal cancers (male HR 0.97; 95% CI: 0.88–1.06; female HR 0.92; 95% CI: 0.83–1.03). The use of menopausal hormonal therapy showed no heterogeneity, meaning there was no difference across anatomical sites (20).
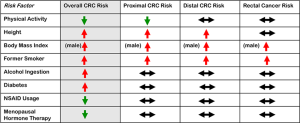
The findings by Murphy et al. suggest a relative CRC risk for anatomical sites within the colon, based on this large prospective cohort study. As summarize in Figure 1, the authors findings suggest that alcohol, diabetes, NSAID use and menopausal hormonal therapy may not be associated with a specific anatomic site for risk, while smoking, male BMI, height, and physical activity could be linked to an anatomic site for risk. In particular, active physical activity lowers proximal CRC risk, taller height increases proximal and distal CRC risks, whereas male BMI and a past history of smoking increase risk for all colon anatomical sites. It is not clear that these lifestyle risks can be linked mechanistically to the anatomic site (e.g., does active physical activity lower microsatellite unstable and/or serrated CRCs?). The authors measured the characteristics at baseline from 1992–2000, and with follow-up for 14.9 years, some parameters could have changed among the individual cases and controls during that time period. Other factors such as diet, microbiome composition, and cancer molecular findings were not assessed, and might have enriched the study. Information regarding morphological features of cancers, such as serrated cancers, might also enhance the findings of the study. The study does not make clear the average age of cancer onset (only the average age of recruitment is noted, with non-cases at 51.2 years of age and CRC cases at 57.3 years of age). This is important to determine if there is any influence from familial CRC cases. The study also makes no mention of any family history. Finally, the study comes from a CRC high risk area, namely Europe, but its findings may not apply to all diverse populations as there is evidence, for example, for some unique genetic driver mutations particularly among African Americans in the U.S. that could be eventually linked to lifestyle conditions (21).
The study by Murphy et al. is the largest study published to link lifestyle factors and anthropometric measurements to the colon anatomical site. Their findings might suggest a connection between the lifestyle factor and the mechanism for CRC initiation as well as the distinct type of cancer at a particular site, which will take much more scientific study. Although current recommendations on lifestyle changes to reduce CRC will not specifically change from the study results, their findings suggest a pathway to explore those connections.
Acknowledgements
Funding: Supported by the United States Public Health Service (R01 CA206010) and the A. Alfred Taubman Medical Research Institute of the University of Michigan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Carethers JM. One colon lumen but two organs. Gastroenterology 2011;141:411-2. [Crossref] [PubMed]
- Takada H, Ohsawa T, Iwamoto S, et al. Changing site distribution of colorectal cancer in Japan. Dis Colon Rectum 2002;45:1249-54. [Crossref] [PubMed]
- Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 2008;135:1079-99. [Crossref] [PubMed]
- Carethers JM, Jung BH. Genetics and genetic biomarkers in sporadic colorectal cancer. Gastroenterology 2015;149:1177-90.e3. [Crossref] [PubMed]
- Carethers JM. Screening for colorectal cancer in African Americans: Determinants and rationale for an earlier age to commence screening. Dig Dis Sci 2015;60:711-21. [Crossref] [PubMed]
- Carethers JM. Systemic treatment of advanced colorectal cancer – tailoring therapy to the tumor. Therap Adv Gastroenterol 2008;1:33-42. [Crossref] [PubMed]
- Carethers JM, Smith EJ, Behling CA, et al. Use of 5-fluorouracil and survival in patients with microsatellite unstable colorectal cancer. Gastroenterology 2004;126:394-401. [Crossref] [PubMed]
- Hamaya Y, Guarinos C, Tseng-Rogenski SS, et al. Efficacy of 5-fluorouracil adjuvant therapy for patients with EMAST-positive stage II/III colorectal cancers. PLoS One 2015;10. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Koi M, Carethers JM. The colorectal cancer immune microenvironment and approach to immunotherapies. Future Oncology 2017;13:1633-47. [Crossref] [PubMed]
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;153:307-23. [Crossref] [PubMed]
- Ashktorab H, Brim H, Kupfer SS, et al. Racial disparity in gastrointestinal cancer risk. Gastroenterology 2017;153:910-23. [Crossref] [PubMed]
- Carethers JM, Stoffel EM. Lynch syndrome and Lynch syndrome mimics: the growing complex landscape of hereditary colon cancer. World J Gastroenterol 2015;21:9253-61. [Crossref] [PubMed]
- Stoffel EM, Koeppe E, Everett J, et al. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018;154:897-905.e1. [Crossref] [PubMed]
- Carethers JM. The increasing incidence of colorectal cancers diagnosed in subjects under age 50 among races: cracking the conundrum. Dig Dis Sci 2016;61:2767-9. [Crossref] [PubMed]
- Satia JA, Keku T, Galanko JA, et al. Diet, lifestyle, and genomic instability in the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev 2005;14:429-36. [Crossref] [PubMed]
- Dono J, Bowden J, Kim S, et al. Taking the pressure off the spring: the case of rebounding smoking rates when antitobacco campaigns ceased. Tob Control 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Murphy N, Ward HA, Jenab M, et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin Gastroenterol Hepatol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Guda K, Veigl ML, Varadan V, et al. Novel recurrently mutated genes in African American colon cancers. Proc Natl Acad Sci USA 2015;112:1149-54. [Crossref] [PubMed]
Cite this article as: Carethers JM. Risk factors for colon location of cancer. Transl Gastroenterol Hepatol 2018;3:76.


