Transplantation for colorectal metastases: on the edge of a revolution
Liver transplantation (LT) for cancer
Beside chronic and acute liver diseases, LT is performed more and more often for malignant indications (1). Selection criteria for transplantation for hepatocellular carcinoma (HCC) have been continuously improved since the initial Milan publication, and more patients can now be included with stable post-transplant outcomes (2-9). Transplantation has also demonstrated a benefit for selected patients with peri-hilar cholangiocarcinoma (10-13), and more recently for patients with very-early intrahepatic cholangiocarcinomas (single <2 cm) (14-16). Considering hepatic metastases, transplantation is a commonly accepted indication for selected patients with neuro-endocrine tumor (17,18). Based on this growing field, the concept of transplant oncology has emerged (19).
One of the upcoming challenges is to determine the place for transplanting patients with colorectal liver metastases (CRLM). The present review is exploring this topic, defining the current state of the field, and extrapolating the future milestones.
CRLM
CRLM are currently the most frequent indication for liver resection in many western countries, and R0 resection is the keystone of their management. Although only 1/3 of the patients are amenable to surgery at the time of the initial presentation, the limits of resectability have been extended over the last 2 decades by several improvements:
- Pro-regenerative manoeuvres allow resecting volumes of liver that would have initially led to post-operative liver failure. The classical procedures are portal vein ligation or embolization, while ALPPS (Associating Liver Partition and Portal vein ligation for Stage hepatectomy) and its modified versions have shown to improve even further the potential for liver regeneration (see Resection for colorectal metastases: to ALPPS or not to ALPPS? In this issue) (20,21);
- The combination of chemotherapeutic agents including biological compounds show a tumor response rate >60% (22-24), and the improved conversion rate toward resection of initially unresectable metastases (25,26). If R0 resection is still the goal to achieve, limited observations suggest that R1 resection leads to similar post-operative overall survival pending that metastases responded to the neo-adjuvant chemotherapy (27,28);
- Multiple repeated liver resections yield improved survival once the metastases have recurred (29), strengthening the concept of liver parenchyma sparing (30);
- Loco-regional treatments, mostly thermal ablation, allow for the destruction of deeply located metastases and limited parenchymal sacrifice during surgery in case of multiple metastases (31).
However, despite this armentarium, some metastases remain technically inaccessible to liver resection, mainly because of the proximity to vital anatomical structures that cannot be sacrificed, and of anticipated insufficient future liver remnant volume. This peculiar situation is the starting point of the concept of LT for CRLM: resecting all metastases (R0) by total hepatectomy.
Historical trials of LT for CRLM
During the eighties, multiple attempts have been made to transplant unresectable liver metastases. In 1991, Mühlbacher et al. published the Vienna experience in LT for metastases, including 17 patients for CRLM, and showing a 5-year survival of 12% with a recurrence rate over 60% (32). These disappointing results were correlated by North American data (33), and it was admitted that the poor post-transplantation survival did not justify using such a scarce resource. Except for limited case reports, no further experience in this field were published until 2013.
The corner: the SECA-I study
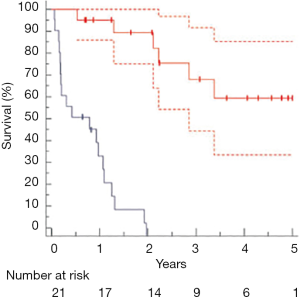
In 2006, Oslo University Hospital started a new protocol of transplantation for CRLM: the SECA-I study (for SEcondary CAncer) (34). This study has been made possible thanks to the peculiar situation of Norway with an excess of liver donors, the average waiting time being approximately 1 month (35). The group from Oslo reported their initial experience with the first 21 transplantations for CRLM: overall survival at 1, 3 and 5 years were 95%, 68% and 60% respectively. Disease-free survival (DFS) was 35% at 1 year and 0% at 2 years (Figure 1). Interestingly, the type of recurrence displayed 2 different patterns with significant differences in impact on survival: 13 patients developed lung metastases only whereas the remainder patients had metastases at multiple sites, including liver. It is worth noting that lung metastases seemed to grow slowly despite of immunosuppression, and several patients could be offered lung resections with curative intent (36). A recent sub-analysis of the patients in the SECA trial who developed lung metastases suggests that the growth of pulmonary metastases is not negatively impacted by immunosuppression (37).
An important part of this study was the inclusion criteria: initially very restrictive, an amendment was accepted after 11 months without patient inclusion. The authors accepted a wide range of clinical characteristics in the study population, the only exclusion criteria being extra-hepatic disease and weight loss >10%. All patients had at least 6 weeks of neo-adjuvant chemotherapy, a complete radical excision of the primary and a good performance status. All patients also received immunosuppression including mTOR inhibitors, mycophenolate and steroids from day one.
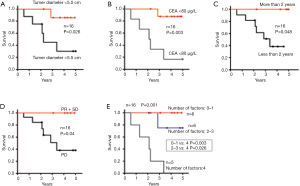
The wide inclusion criteria allowed the identification of 4 clinical features associated with a worse survival: pre-transplant tumor diameter >5.5 cm, a pre-transplant CEA >80 µg/L, time interval from resection of the primary to transplantation <2 years, and progression of the metastases under neo-adjuvant chemotherapy (Figure 2). These four features were integrated into the Oslo Score, which is the mathematical addition of each factor (each factor representing 1 point).
Questions raised by the SECA-I study, partial answers and how to give a final response
The results of the SECA study raised several questions that can be summarized below.
Is it reasonable to consider LT for CRLM in the current context of organ shortage?
This utilitarian question has no clear answer, but we can approximate a response by comparing the results of LT for other accepted common indications. There is no universal definition of the minimal survival requirements after a LT: ranges between 50% and 70% have been suggested (38). In the publication that has set the gold standard of transplantation for HCC, Mazzaferro et al. have set the survival at 70% at 5 years (1). In the 2018 report of the SRTR, the mean graft survival among adult deceased donor liver transplant recipients was approximately 75% at 5 years. However, the 5 years survival dropped to 70% or even below for recipient ≥65 years old, for recipients transplanted for HCV (before the era of new antiviral drugs) or HCC, and almost reached 60% for patients who underwent retransplantation (39). The question should therefore be: why deny LT for CRLM if we accept retransplantations with a similar 5-year survival rate? An argument against transplantation for CRLM is its associated high recurrence rate, and the expected sharper drop in survival beyond 5 years.
However, a recent report from the Compagnons Hepato-Biliaires including 12 patients from 4 European centers showed that 5 transplanted patients were alive without evidence of recurrence at the time of the writing of the manuscript with follow-ups of 7, 43, 47, 48, and 108 months after transplantation (40). Although the overall survival was lower at 4 years than the Oslo series (50%), this report demonstrated that a prolonged survival without recurrence is possible in selected patients. Moreover, in the experience of the Compagnons Hepato-Biliaires, some patients received a “compassionate” or urgent transplantation (as opposed to planned transplantation) and this was associated with a dismal survival.
Overall, the reported outcome of the SECA study is related to a relatively unselected population, and it is likely that improved results can be obtained by refined selection criteria. At the present time, all candidates for LT for CRLM should probably be enrolled in a trial. A SECA-II trial (NCT01479608) has already started and selects patients based on the relevant variables of the SECA-I study: please see below. Another ongoing trial is the Porto Alegre protocol of the Compagnons Hepato-Biliaires that also selects patients according variables to similar to the SECA-I, but also includes a molecular analysis of the tumor (see below) and includes adjuvant chemotherapy. These 2 trials aim at determining the overall and DFSs of carefully selected patients.
Is transplantation better than chemotherapy for matched patients?
As opposed to the first question, this one exposes the role of LT at the level of the individual patient. Again, a preliminary answer can be inferred from the results of the SECA-I study. Dueland et al. compared the results of the 21 patients transplanted in the SECA-I study, to 47 similar patients enrolled in a palliative treatment based on Nordic FLOX for unresectable liver only colorectal metastases (NORDIC VII study) (41). The results showed a significantly better survival at 5 years for the transplanted patients compared to the chemotherapy group (56% vs. 9%, P<0.001), although the DFS was similar. However, a definitive advantage of LT cannot be considered based on this report alone, as biases exist related to the retrospective nature of the study.
A prospective French study randomizing patient with unresectable CRLM to LT or best palliative chemotherapy is ongoing under the name of TRANSMET (NCT02597348).
Another prospective trial from Oslo, called SECA-III, will also randomize patients with unresectable CRLM to LT or best alternative treatment (including chemotherapy and locoregional treatments) (NCT03494946). These trials should provide definitive answer to the question.
Who is the ideal candidate for transplantation for CRLM?
The answer is balanced between the needs of the individual and the needs of the population. At an individual level, the best option between transplantation and alternative treatments (chemotherapy) will depend on the results of the TRANSMET and SECA-III studies. If these studies confirm a clear survival advantage for transplantation, one could be tempted to even offer it to patients with a predicted survival inferior to 60% at 5 years, provided that the survival in the chemotherapy group is worse. At a population level, and accounting for the graft shortage, transplantation programs should select patients with a reasonable anticipated survival, the level of which still needs to be defined. This task is made more difficult by the fact that only few models are able to generate a calibrated predicted survival (42). Regional and national differences in waiting times, wait list mortality and epidemiological burden of chronic liver diseases is apparent.
Concerning the variables, the 4 factors associated with better overall survival in the SECA-I study were: size <5.5 cm, time interval between the diagnosis of the primary and the LT >2 years, CEA <80 µg/L and stability or regression of the metastases on neo-adjuvant chemotherapy. The study of the Compagnons Hepato-Biliaires also showed a better outcome with a time interval >2 years and a CEA <80 mg/L, and demonstrated that “compassionate” LT is associated with poor outcome (40).
A retrospective study using data from the SECA study also showed that the Metabolic Tumor Volume and the Total Lesional Glycolysis of all the CRLM measured on 18F-FDG PET/CT was a predictor of post-transplantation survival (43). Due to its high sensitivity to detect extra-hepatic metastases, PET/CT is certainly becoming a mandatory step in the candidate selection process (44).
At a population level, we can assume that the ideal candidates are those who do not present the risk factors described above. A recent report from the group of Oslo retrospectively compared the outcome of the patients in the SECA-I study who had all 4 risk factors versus patients with 0 to 3 risk factors (Oslo score 4 vs. 0–3) (45). They found that the overall 5-years survival was 75% in the low-risk group versus 0% in the high-risk group. Moreover, patients presenting the 4 unfavourable variables recurred earlier and had a decreased post recurrence survival.
Concerning the biological factors of the tumour, the French group from TRANSMET excluded patients with mutated BRAF, which is a well-known unfavourable prognostic factor of primary and metastatic colorectal cancer (46). The Porto-Alegre protocol also excludes these patients.
The ongoing trials SECA-II and Proto Alegre protocol select patients based on low risks, and should answer the question of the survival of the best candidate at the population level.
Should we consider LT for patients with resectable CRLM?
This is “terra incognita”, as no series have reported such treatment. The current gold standard treatment is an R0 surgical resection whenever possible. Altogether, the overall survival reported in large series such as the LiverMetSurvey that encompasses >25,000 liver resections for CRLM, ranges between 40% and 50% at 5 years, and drops below 40% for patients presenting >3 CRLM (47). In the SECA-I study the overall survival was around 60% at 5 years and the patients presented median 8 CRLM (range, 4–40). We can therefore speculate that, at least for some very selected patients with a large tumor burden, transplantation might be a better option than resection in term of overall survival. However, it has to be emphasized that patients currently transplanted for CRLM have very stable disease under chemotherapy, and are certainly not representative of patients enrolled in large databases. We recommend extreme caution for this particular indication, and transplant criteria cannot be extrapolated from the recent experience.
How can we supply a potential high demand?
We can postulate that the studies mentioned above will demonstrate a benefit of transplantation over any alternative treatment for selected patients. As CRLM is currently the main indication for liver resection in most of the Western world, the demand could be potentially high. On the other hand, post-transplant survival could be slightly inferior compared to other indications, and this will aggravate the imbalance in distributional fairness of a scarce resource. Some stategies have emerged to solve this important issue.
The group of Oslo developed a procedure aiming at using only left lateral segment graft as a surplus liver. This complex procedure, called the RAPID concept, takes advantage of the normal function of the non-tumour parenchyma (48). During the first step, only segments 1 to 3 are resected in the recipient to provide space and the left lateral split graft transplanted. After revascularization, a clamping test of the right branch of the portal vein is performed. It is ligated if the portal pressure does not exceed 20 mmHg. If the pressure exceeds 20 mmHg several steps (splenic artery ligation, banding instead of ligation of portal vein, portocaval shunt) are undertaken sequentially to reduce the portal pressure in the liver graft, in order to avoid a small for size syndrome. The volume of the graft is then monitored weekly, and a completion stage 2 hepatectomy of the right liver remnant is performed once the volume of the transplanted graft reaches 0.8% of body weight or 40% of recipient standard liver volume.
The RAPID concept has been initially reported in one single case, and two studies are currently analysing the feasibility of this procedure at a larger scale:
- The extension of the case report into a prospective trial by the group of Oslo (NCT02215889);
- The LIVERT(W)OHEAL study from Jena University Hospital and University Hospital Tuebingen in Germany (NCT03488953): this study will apply the RAPID concept to living donors.
Using living donors may also represent a valid approach to increase the pool of liver grafts. This alternative allows transplanting patients with CRLM without prejudice to other candidates for LT. Beside the Jena LIVERT(W)OHEAL study that uses auxiliary grafts from living donors, the Toronto General Hospital started a non-randomized prospective trial of full living donor grafts (not auxiliary) for patients with unresectable CRLM limited to the liver and not progressing under neo-adjuvant chemotherapy (NCT02864485). Post-transplant outcomes will be compared to patients who dropped out of the protocol for non-cancer progression reasons.
The timing of chemotherapy?
The above-cited protocols [SECA I-II-III, TRANSMET, RAPID, Porto Alegre, LIVERT(W)OHEAL, Toronto protocol] use neo-adjuvant chemotherapy. All patients are deemed unresectable and should receive at least an attempt of conversion chemotherapy. Another reason to use neo-chemotherapy is that the SECA-I trial showed a worse outcome for patients progressing on chemotherapy (34,49) before transplantation.
A more debated point is whether or not to give post-transplant chemotherapy. In the SECA-I study, patients did not receive chemotherapy, and post-transplantation chemotherapy is not routinely given for patients enrolled in the SECA II and III, as well as in the RAPID trial. The underlying rationale is that most of the patients transplanted for CRLM already have received extensive chemotherapy and many of them received a second or even a third line, a significant proportion of them experiencing side effects. In this context, the choice of chemotherapy regimen and the potential benefit are not obvious. Moreover, post-transplantation chemotherapy might hamper liver regeneration, which is particularly relevant in the RAPID protocol and in the SECA-III study in which extended donor criteria grafts are to be used.
In the TRANSMET protocol, patients are receiving a limited post-operative chemotherapy. Any significant improvement of the survival during the period of adjuvant chemotherapy can be imputed to transplantation, as transplantation is the only significant modified variable between the two groups. In the trial from Toronto, patients receive a post-transplant standard-of-care chemotherapy based on FOLFOX or FOLFIRI with or without bevacizumab.
As adjuvant chemotherapy will probably not impact the survival as significantly as the transplantation itself, the results of the current ongoing trials are certainly mandatory prior to analyze this specific point with a randomized study.
Conclusions
The recent published data on transplantation for CRLM have shown promising results and raised several questions, giving birth to multiple randomized trials trying to answer them.
Preliminary data of the SECA-II trial have been recently presented at the 2018 annual congress of the International Liver Transplant Society in Lisbon. For 15 patients transplanted within Oslo score of 0–1, overall 5-years survival was comparable to HCC patients transplanted within the Milan criteria and non-malignant indications for LT. Although DFS did not reach the same level, refined criteria allowed to further improve DFS, and the majority of patients who recurred were amenable to curative-intent surgical resection and obtaining status of no evidence of disease.
These results confirm the anticipated hypothesis that survival results similar to commonly accepted indications can be obtained for selected patients with unresectable CRLM. We can therefore reasonably anticipate that ongoing trials will show that transplantation is superior to any alternative treatment for unresectable CRLM. The optimal selection criteria are still not fully established. The group of Oslo has set a score allowing inclusion of patients with a favourable outcome, but other trials are still working at defining ideal inclusion criteria. Current criteria are restrictive, given the limited international experience in LT for CRLM and the recurrence rate that is higher compared to other oncological indications. However, as the group of Oslo demonstrated that in many cases recurrent disease is accessible to a curative-intent resection, it should maybe be anticipated in the global assessment of the transplant candidate. Besides, we should not lose sight of the primary goal, which is the overall survival. The experience showed that immunosuppression has a limited impact on the course of metastases and we should probably adapt our appraisal of the DFS in this context.
Considering the numerous potential candidates, we are certainly at the dawn of a revolution in LT, and one should remain extremely cautious analysing the results of the upcoming studies in order to define best practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009;250:141-51. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Toso C, Asthana S, Bigam DL, et al. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology 2009;49:832-8. [Crossref] [PubMed]
- DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-72. [Crossref] [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3; quiz e14-5.
- Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015;62:158-65. [Crossref] [PubMed]
- Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018;154:128-39. [Crossref] [PubMed]
- Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98.e3; quiz e14.
- De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl 2000;6:309-16. [Crossref] [PubMed]
- Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis 2004;24:201-7. [Crossref] [PubMed]
- Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant 2002;2:774-9. [Crossref] [PubMed]
- Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016;64:1178-88. [Crossref] [PubMed]
- Sapisochin G, Rodriguez de Lope C, Gastaca M, et al. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant 2014;14:660-7. [Crossref] [PubMed]
- Lunsford KE, Javle M, Heyne K, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol 2018;3:337-48. [Crossref] [PubMed]
- Mazzaferro V, Sposito C, Coppa J, et al. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases From Neuroendocrine Tumors. Am J Transplant 2016;16:2892-902. [Crossref] [PubMed]
- Le Treut YP, Gregoire E, Klempnauer J, et al. Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: a 213-case European liver transplant registry study. Ann Surg 2013;257:807-15. [Crossref] [PubMed]
- Hibi T, Itano O, Shinoda M, et al. Liver transplantation for hepatobiliary malignancies: a new era of "Transplant Oncology" has begun. Surg Today 2017;47:403-15. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Lang H, de Santibanes E, Schlitt HJ, et al. 10th Anniversary of ALPPS-Lessons Learned and quo Vadis. Ann Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609-18. [Crossref] [PubMed]
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306-15. [Crossref] [PubMed]
- Cremolini C, Loupakis F, Masi G, et al. FOLFOXIRI or FOLFOXIRI plus bevacizumab as first-line treatment of metastatic colorectal cancer: a propensity score-adjusted analysis from two randomized clinical trials. Ann Oncol 2016;27:843-9. [Crossref] [PubMed]
- Tomasello G, Petrelli F, Ghidini M, et al. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA Oncol 2017;3. [Crossref] [PubMed]
- Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509-20; discussion 520-2. [Crossref] [PubMed]
- de Haas RJ, Wicherts DA, Flores E, et al. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg 2008;248:626-37. [PubMed]
- Ayez N, Lalmahomed ZS, Eggermont AM, et al. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol 2012;19:1618-27. [Crossref] [PubMed]
- Wicherts DA, de Haas RJ, Salloum C, et al. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg 2013;100:808-18. [Crossref] [PubMed]
- Andres A, Majno PE, Morel P, et al. Improved long-term outcome of surgery for advanced colorectal liver metastases: reasons and implications for management on the basis of a severity score. Ann Surg Oncol 2008;15:134-43. [Crossref] [PubMed]
- Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009;197:728-36. [Crossref] [PubMed]
- Mühlbacher F, Huk I, Steininger R, et al. Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transplant Proc 1991;23:1567-8. [PubMed]
- Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery 1991;110:726-34; discussion 734-5. [PubMed]
- Hagness M, Foss A, Line PD, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg 2013;257:800-6. [Crossref] [PubMed]
- Scandiatransplant.org. Liver Annual Report. Annual2016.
- Hagness M, Foss A, Egge TS, et al. Patterns of recurrence after liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg Oncol 2014;21:1323-9. [Crossref] [PubMed]
- Grut H, Solberg S, Seierstad T, et al. Growth rates of pulmonary metastases after liver transplantation for unresectable colorectal liver metastases. Br J Surg 2018;105:295-301. [Crossref] [PubMed]
- NHSBT. Liver Transplantation: Selection Criteria and Recipient Registration, 2018. Available online: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/9440/pol195_7-liver-selection-policy.pdf
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant 2018;18 Suppl 1:172-253. [Crossref] [PubMed]
- Toso C, Pinto Marques H, Andres A, et al. Liver transplantation for colorectal liver metastasis: Survival without recurrence can be achieved. Liver Transpl 2017;23:1073-6. [Crossref] [PubMed]
- Dueland S, Guren TK, Hagness M, et al. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann Surg 2015;261:956-60. [Crossref] [PubMed]
- Andres A, Montano-Loza A, Greiner R, et al. A novel learning algorithm to predict individual survival after liver transplantation for primary sclerosing cholangitis. PLoS One 2018;13. [Crossref] [PubMed]
- Grut H, Dueland S, Line PD, et al. The prognostic value of (18)F-FDG PET/CT prior to liver transplantation for nonresectable colorectal liver metastases. Eur J Nucl Med Mol Imaging 2018;45:218-25. [Crossref] [PubMed]
- Grut H, Revheim ME, Line PD, et al. Importance of 18F-FDG PET/CT to select patients with nonresectable colorectal liver metastases for liver transplantation. Nucl Med Commun 2018;39:621-7. [Crossref] [PubMed]
- Dueland S, Foss A, Solheim JM, et al. Survival following liver transplantation for liver-only colorectal metastases compared with hepatocellular carcinoma. Br J Surg 2018;105:736-42. [Crossref] [PubMed]
- Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med 2009;361:98-9. [Crossref] [PubMed]
- Adam R. LiverMetSurvey, Statistics December 2015, .www.livermetsurvey.org
- Line PD, Hagness M, Berstad AE, et al. A Novel Concept for Partial Liver Transplantation in Nonresectable Colorectal Liver Metastases: The RAPID Concept. Ann Surg 2015;262:e5-9. [Crossref] [PubMed]
- Dueland S, Hagness M, Line PD, et al. Is Liver Transplantation an Option in Colorectal Cancer Patients with Nonresectable Liver Metastases and Progression on All Lines of Standard Chemotherapy? Ann Surg Oncol 2015;22:2195-200. [Crossref] [PubMed]
Cite this article as: Andres A, Oldani G, Berney T, Compagnon P, Line PD, Toso C. Transplantation for colorectal metastases: on the edge of a revolution. Transl Gastroenterol Hepatol 2018;3:74.


