Resection for Klatskin tumors: technical complexities and results
Introduction
Definition
William Altemeier in 1957 (1) and Gerald Klatskin in 1965 (2) were the first who described cholangiocarcinoma (CCA) as tumor entity arising between the bile ducts (BDs)’ confluence and the insertion of the cystic duct into the common BD. Klatskin tumors have been redefined as perihilar CCA (phCCA) and constitute 50–70% of all CCAs (3-6).
Epidemiology
phCCA develops in a context of chronic inflammation and cholestasis usually secondary to:
- PSC (primary sclerosing cholangitis);
- Liver flukes (mainly spread in Asiatic countries);
- Hepatolithiasis;
- Caroli’s disease;
- Congenital hepatic fibrosis;
- Choledochal cysts;
- Viral hepatitis B and C infection;
- Liver cirrhosis;
- Chemical compounds—dioxin, thorotrast;
- Obesity and diabetes.
However, epidemiological reports are heterogeneous because, on the one hand, the definition and classification of the tumor entity has undergone several changes in recent decades (7). On the other hand, there are significant regional differences, particularly between the United States and Europe compared to Asian countries (8). The latter seems to reflect the geographical distribution of environmental and genetic predisposing influences for the development of CCA (9). The incidence of phCCA increases with age; usually, the tumor occurs between 60 and 70 years of age (10).
Growth pattern
phCCA are usually adenocarcinomas arising from periductal glands located in the intra- or extrahepatic BD epithelium (11).
According their growth pattern the Liver Cancer Study Group of Japan proposed three different type of phCCA (4): (I) intraductal; (II) periductal or (III) mass forming.
Additionally, phCCA may grow longitudinally (into the liver) or radially (into adjacent structures) (12).
Classification and staging systems
An optimal staging system should provide information about prognosis, guide the therapy and allow their comparison. Different classifications were proposed for phCCA. We identified two types of classifications: surgical and oncological. The first type is based on preoperative imaging and it is useful in planning the operation but does not correlate well with prognosis, whereas the second one provides better information about prognosis, but is limited by the need of histology. Therefore, it could be applied only postoperative.
Surgical classification
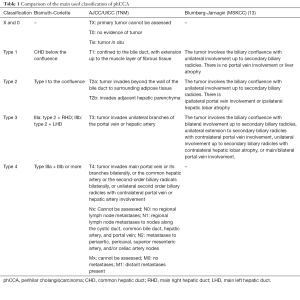
The most famous surgical Bismuth-Corlette (1975) classification differentiates four types of tumor focusing just on BD invasion (see Table 1) (14). Although old, it is still nowadays the most used classification that easily depicts the phCCA at its presentation.
Full table
Despite the close correlation between tumor localization within the biliary confluence and portal vein (PV) or hepatic artery (HA) infiltration, this classification does not provide any information about circumferential extension as well as local or distal metastases.
Oncological classification
From oncological point of view the following four factors have been reported as main prognostic ones:
- Extension of tumour within the biliary tree;
- Vascular invasion;
- Lobar atrophy;
- Metastatic disease.
However, other aspects as lymph node metastases, tumor differentiation, perineural invasion, surgical margins are well known independent prognostic factors in phCCA (13,15). For this reason, oncological classifications consider also local and distal invasion.
In this context, two major staging systems are commonly used: (I) the American Joint Cancer Committee/Union Internationale Contre le Cancer (AJCC/UICC) and (II) the Memorial Sloan-Kettering Cancer System (MSKCC) (see Table 1).
The AJCC/UICC is constantly reviewed to improve it prognostic predictive power and it arises the best once histology is obtained (16). Starting from the 7th edition of the AJCC/UICC staging system the phCCA is an independent entity (see Table 1). The major limit is that it can be used only in resected patients (17,18).
On the other hand, the MSKCC system mixes histology and imaging aspects like tumor extension, portal venous invasion, and hepatic lobar atrophy (13,19). For this reason, the MSKCC can be useful also preoperatively to plan the global therapeutical strategy. However, it does not provide information about metastasis.
Recently, two additional classifications, still in need of validation, have been proposed: (I) the Mayo Clinic Classification and (II) the De Olveira Classification.
The Mayo Clinic classification includes additional factors such as the size and multifocality of the primary tumour, the nodal and extraregional metastatic burden, and clinical features such as jaundice and performance status (20).
The DeOliveira classification considers following aspects (21): (I) BD invasion; (II) tumor size; (III) tumor form (F); (IV) more than 180° of involvement of the HA; (V) more than 180° of involvement of the PV; (VI) liver remnant volume; (VII) underlying liver disease (D); (VIII) lymph nodes; (IX) metastasis.
The DeOliveira classification does represent the sum of the most important surgical aspects, which should be considered when planning an operation for phCCA.
Role and limits of surgery
Surgical resection provides the only chance of cure for this disease but is technically challenging because of the complex, intimate and variable relationship between biliary and vascular structures at this location (19,22,23).
Resectability ranges from 32% to 80%, but surgical margins are microscopically involved in 20–30% of patients who undergo resection (13,15,23-30). R0 resection is linked to improved survival, and major hepatic resection including caudate lobectomy is necessary to obtain clear longitudinal and radial margins (13,15,23-30).
Five-year survival rates range between 25–45% (median 27–58 months) in case of R0 resection and 0–23% (median 12–21 months) in case of R1 resection respectively (31,32).
The major costs of high radicality are represented by relatively high morbidity and mortality rates (i.e., 20–66% and 0–9% respectively) (13,15,23-30).
Aim
Considering that radical resection may represent the only curative treatment of phCCA, we focused our review on surgical planning and techniques that may improve resectability rates and outcomes for locally advanced phCCA (i.e., stage III and IV tumors according the Bismuth classification, with biliary extension into both hepatic ducts, bilateral extension to second order BDs or vascular involvement).
Surgical strategy
The surgical treatment of phCCA can be successful when following aspects have been fulfilled:
- Accurate preoperative diagnostic aimed to identify the tumor in all its details (localization and extension) and to study all the risk factors influencing a posthepatectomy liver failure (PHLF): i.e., liver volume, liver function, liver quality, haemodynamics and patient characteristics;
- Precise surgical technique taking in consideration the local extension of the tumor and the vascular invasion;
- Adequate postoperative management aimed to avoid major complications (i.e., PHLF and biliary complications).
Preoperative diagnostic
The primary role of preoperative diagnostic is to determine (or exclude) the diagnosis of phCCA and its staging in terms of spread into the BDs, the liver parenchyma, PV and HA invasion, as well as metastatic disease. Secondarily the liver anatomy, volumetry and functionality should be also preoperatively investigated.
Although a lot different radiological and endoscopically methods are available, the diagnosis of phCCA remains challenging, due to lack of sensibility or specificity of the different methods. For this reason, a combination of the various procedures should be considered.
Imaging
Triple phase computed tomography (CT)
While conventional CT has a low accuracy, high-resolution CT can precisely predict resectability in most phCCA. Despite it plays a main role in determine the vascular involvement and metastatic disease, in some cases it could lack in delineate the BD invasion and detect low volume peritoneal metastases or nodal disease (33-36). Therefore, it should be always accompanied by an MRI.
Magnetic resonance cholangiopancreatography (MRCP)
MRCP and particular contrast-enhanced magnetic resonance cholangiography is the tool of choice to determine the biliary extend of phCCA, associated lobar atrophy and loss of liver volume with a sensitivity of 94% and specificity of 100% (37). It is also able to determine vascular inflow. The use of contrast-enhanced MRI (CE-MRI) with gadolinium-based contrast agents (Gd-EOB-DTPA) allows also more accurate depiction of benign or malignant liver lesions than CT (38-41).
CT and MRCP should be always combined (39).
Positron emission tomography (PET)
The use of PET in phCCA is still debated. It shows a low sensitivity for the diagnosis of primary lesion, as phCCA, like the non-malignant tumors, are not fludeoxyglucose (FDG)-avid (19,39,42,43).
Endobiliary procedures
Cholangiography
A percutaneous or endoscopic cholangiography has the capacity of a better visualization of the BDs. It is reported a sensibility of 84% and specificity of 97% (37). Considering that a lot of patients will undergo a biliary stent placement, it is an easy additional information also about anatomy of intrahepatic biliary tree (44). However, these techniques are associated with a risk of cholangitis as well as tumor dissemination (33,45).
Endosonography (EUS)
EUS can determine the involvement of extraregional lymph nodes and, in case of intraductal ultrasound (IDUS) also the BDs and periductal tissue can be examined, raising the accuracy of the examination up to 90% (46,47).
Brush cytology
Brush cytology has an excellent specificity but poor sensitivity. Moreover, a definitively positive result is achievable in just 40% of patients. Therefore, a negative result does not exclude a phCCA (48).
Cholangioscopy
Cholangioscopy allows a direct visualization of BD and therefore enables guided biopsies, increasing sensitivity and specificity to detect the phCCA (49-51).
Biopsy
Percutaneous or laparoscopic biopsy is not recommended as it has low sensitivity and increases risk of tumour dissemination (39,52).
Explorative laparoscopy
As mentioned above, CT and MRI imaging lack in detecting low volume peritoneal metastases. Despite the improvement of imaging sensibility increased in the last years (23,53-55), 20% to 50% of patients still present liver or peritoneal metastatic disease at the time of surgical exploration (13,19,56,57). Explorative laparoscopy, with opening of the lesser sac and examination of the common HA and its lymph node, has been proposed to detect occult metastasis and prevent the patient an unneeded laparotomy. This method can reveal up to 45% of the metastasis with an accuracy of 32–72% (33,43,58-61).
Preoperative prophylaxis of PHLF
According to the International Study Group of Liver Surgery (ISGLS), PHLF is defined as “a post-operatively acquired deterioration in the ability of the liver to maintain its synthetic, excretory, and detoxifying functions, which are characterized by an increased INR and concomitant hyperbilirubinemia on or after postoperative day 5” (62). It is the most feared complication occurring after liver resection with volumetric or functional insufficient future remnant liver (FRL). Its clinical presentation ranges from slight hepatic insufficiency to liver and multiorgan failure with requirement of intensive care.
For this reason, every patient with a suspected phCCA should be presented to a high-volume center to undergo a precise diagnostic, selection and therapeutical decision to maximize the oncological outcome and minimize the operative risks.
Following factors do usually influence the occurrence of a PHLF: (I) patient’s characteristics/comorbidities; (II) FRL volume; (III) liver quality; (IV) liver function; (V) inflow and outflow
Patient assessment
Patients with suspected phCCA usually present with painless jaundice, pale stools and dark urine. Lab values shows the haematological hallmarks of obstructive cholestasis (33,63).
Mortality seen after PHLF is likely multi-factorial and many patient-related factors play a main role. Diabetes, obesity, malnutrition and frailty, hepatitis, renal insufficiency, comorbidities and age older than 65 years are associated with PHLF (64-71). Miscellaneous scores, like Karnofski and ECOG performance status or the Charlson Comorbidity Index, are used to assess the global patient status, but there is no agreement in the literature. Few studies also suggested performance status, comorbidities, and albumin serum concentrations as prognostic factors (72,73). Some mortality risk scores were proposed (69,74), however a validation is still needed.
Still, improving the patient’s condition at the time of surgery is mandatory to optimize the outcome.
Liver assessment
Volumetry
Different studies showed that the volumetric assessment of FRL correlates with remnant liver function and the risk of PHLF (75). It could be defined as future liver remnant volume to total liver volume percentage (TLV/FRL) or to body weight, known also as rest volume to body weight ratio (RVBWR) (76,77).
Following ranges have been suggested: TLV/FRL >25% or RVBWR >0.5% in patients with a normal liver, or up to >30–40% and 0.8% in patients with cholestasis or suspected poor liver quality (75,78-86).
Volumetry can be assessed either manually, semi-automatic or automatic with software-assisted image postprocessing liver volumetry (SAIP) (87-89) and can be both CT- or MRI-based (90-93).
However, an interdisciplinary work between hepato-biliary-pancreas (HBP)-surgeon and radiologist is mandatory in order to determine the cut line in case of complex resection (e.g., extended left).
Liver quality
The performance of FRL is not only a matter of liver volume but it is directly related to the quality of the liver parenchyma, which in turn is mainly dictated by underlying diseases such as fibrosis, steatosis or cirrhosis (94-97).
Liver quality can be assessed preoperatively by means of following procedures:
Biopsy
Liver biopsy represents the gold standard, as can provide exact information about liver quality (95). However, it could be associated with false negative results due to sampling errors and it is an invasive technique that can be associated to complications as bleeding or infections (52,98-100).
For this reason, the development of non-invasive techniques, particularly elastography and MRI is to be preferred preoperatively.
Ultrasonography
High frequency ultrasound is a feasible and inexpensive tool that can suggest a poor liver quality due attenuation parameters (101). However, it shows a moderate sensitivity and need of clinician expertise (102).
Elastography
Several ultrasound elastography techniques have been developed to detect liver fibrosis [transient elastography, real time elastography (RTE), acoustic radiation force impulse imaging (ARFI) and shear wave elastography (SWE)]. The European Federation of Societies for Ultrasound in Medicine and Biology guidelines suggests that values above 6.8–7.6 kPa indicate the presence of significant fibrosis and that those ranging between 11.0–13.6 kPa may indicate cirrhosis (103). Promising results using elastography to predict PHLF were already published (104-106).
MRI
As conventional MRI can assess just indirect information in case of cirrhosis or portal hypertension, a lot of different MRI-based techniques [MR elastography, Diffusion-weighted MR imaging (DWI), gadoxetic acid disodium (Gd-EOB-DTPA)] are now available to assess hepatic steatosis and fibrosis and the results are comparable to US-based elastography techniques.
Moreover, these MRI-based techniques are the most accurate for measuring liver fat content and, contrary to US-based imaging, are feasible also in obese patient or with ascites, and allow an evaluation of the whole liver (107-110).
On the other side, these methods are expensive, associated to long examination time, patient compliance and could be limited by hepatic iron overload, vascular and biliary congestion (110-113).
MRI allows the segmental assessment of steatosis and can be used to assess fibrosis, making it a potential one-stop-shop modality for both liver anatomy as well as function.
CT
Liver attenuation obtained with CT-scan, compared with that observed in the spleen can indicate hepatic steatosis (75,114). However, CT has a low sensitivity in detecting fibrosis.
Liver function
The volumetric assessment of the liver should be complemented by liver function-specific assays. Various methods were proposed. Most of them can assess the global function of the liver based on blood assays and new imaging procedures (i.e., functional scintigraphy or MRI) are able to assess the segmental liver function.
Biochemistry
Various laboratory parameters show the synthetic or extraction capacity of the liver. They include coagulation parameters [prothrombin time (PT)/Quick/INR as well as coagulation factors], protein (albumin, total protein), cholinesterase (CHE) (115,116) and cholestasis parameters (bilirubin, gGT, ALP). Different series showed inconsistent findings as predictors of PHLF when taken singularly or in association as in the ISGLS score (117-121).
In fact, these parameters can be influenced from several other factors (i.e., loss, deficiency state, substitution) as well as by other diseases such as systemic inflammation, the nephrotic syndrome, malnutrition, or protein-losing enteropathy.
Indocyanine green clearance test (ICG)
The ICG test is the worldwide most use test in liver surgery (122). It is based on the capacity of ICG to be excreted, after intravenous administration, exclusively by the liver without biotransformation (123,124).
After intravenous administration the ICG plasma disappearance rate after 15 minutes can be measured by pulse spectrophotometry (ICG-15) (125). The safety limit in predicting safe liver resection of ICG-15 varies in the different studies from 15% to 20% (126-130).
However, in up to 20% of patients the severity of liver disease is underestimated due to hyperbilirubinemia, as the uptake is facilitated by common hepatic transporters, and impaired blood flow, as in case of intrahepatic shunting (129).
13C-methacetin breath test (LiMAx)
The LiMAx breath test is based on the metabolism of 13C-methacetin by the liver cytochrome CYP1A2. It assesses the global liver function but the authors suggest using the percentage of FLR to TLV as the percentage of functionality of the FRL. The normal cutoff value is set at 311–575 µg/kg/h (131). Notwithstanding, this ignores the lacking uniformity of the liver function throughout the liver (122). Moreover, besides the slightly availability of the device, different factors as smoking, nutrition and visceral hemodynamics can affect the results (132).
Hepatobiliary scintigraphy (HBS)
Hepatobiliary scintigraphy (HBS) can finally show the regionality of the liver function. The most discussed are 99mTc-galactosyl serum albumin scintigraphy (99mTc-GSA) and 99mTc-mebrofenin hepatobiliary scintigraphy (HIDA), that shows respectively the uptake and excretion capacity of the liver.
99mTc-GSA
99mTc-GSA is uptake only in the liver and is unaffected by hyperbilirubinemia (133). Combined with dynamic single photon emission CT (SPECT-CT) allows an accurate three-dimensional measurement of FRL preoperatively also in cholestatic patients (134,135).
Various studies already shown that the uptake ratio of FRL correlate well with postoperative liver function parameters and this method can be used to predict postoperative outcome (135-141).
The applicability of 99mTc-GSA SPECT-CT in monitoring FRL after PV embolization (PVE) has been evaluated several times. The increase in FRL function after PVE was more pronounced compared to the volumetric increase measured with CT volumetry (142,143).
Three-dimensional SPECT-CT provides additional adequate anatomical information (144).
99mTc-HIDA
Mebrofenin is a lidocaine analogue that similar to ICG, is uptaken and excreted from the liver without undergoing any biotransformation (145-147). Moreover, 99mTc-mebrofenin shows the lowest displacement by bilirubin in case of hyperbilirubinemia. For this reason it is particularly indicated in cholestatic diseases (148).
As the results are similar to ICG clearance test (149), HIDA correlates with postoperative FRL function and allows a segmental view of it (147,150-153). A 99mTc-mebrofenin uptake in the FRL <2.69%/min/m2 is associated to a high postoperative liver failure (152,154).
99mTc-mebrofenin HBS with SPECT-CT is gaining applicability in monitoring regeneration after PVE or liver resection. Recent reports have indicated that the increase in FRL function is more pronounced than the increase in FRL volume (144,150). This finding suggests that the time interval between PVE and liver resection should not be determined by volumetric parameters alone.
MRI with gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)
Gd-EOB-DTPA is a liver-specific contrast agent. Approximately 50% is excreted by hepatocytes and the rest by the kidneys.
First proposed in 1993, data on the assessment of liver function using MRI with Gd-EOB-DTPA confirmed the possibility of segmental liver function assessment using MRI (155-163).
In- and out-flow assessment
In- and out-flow assessment implies not only the liver anatomy but also the regional territorial liver mapping, functional volumes, and outflow congestion volumes (164,165).
A three-dimensional computer-assisted surgical planning (3D-CASP) software could be helpful to determine the resection plane (164,166). 3D-CASP is based on information derived from CT and MRI and can provide inflow and outflow virtual analyses as well as determine safely perfused and drained retained liver volumes. In case of extended liver resection, knowing that outflow obstruction could lead to PHLF (167,168), this technique could contribute to provide information about the middle hepatic vein territory (169).
Augmentation techniques
If the risk of PHLF is set, while planning an extended hepatectomy, augmentation techniques to increase the volume and the function of the FRL of can be considered. These includes PVE and associating liver partition and PV ligation for staged hepatectomy (ALPPS).
PVE
Embolisation of main PV branches induces, due to deviation of the total PV flow to the still perfused liver, hypertrophy of the remnant liver (170,171). It is reported an average increase of the FRL in 3 weeks between 8–46% (80,172).
A new assessment of volumetry and functionality, as above described, should be assessed 3–4 weeks after PVE to determine the degree of hypertrophy (171). Besides the already mentioned volumetric and scintigraphic parameters, a degree of hypertrophy >5% is associated with improved patient outcomes (173).
This procedure is associated with low morbidity and very low mortality, even though cholangiosepsis is reported (53,80,172,174,175). In planning of an extended right resection the PVE of segment 4 should be also considered, if technically feasible (176). In selected cases with insufficient hypertrophy of the FRL, an additional embolization of the ipsilateral hepatic vein could be considered to force the FRL growth (177,178). Transhepatic ipsilateral approach should be preferred to contralateral approach to avoid an injury of the FRL.
The resectability of the FRL is achieved in more than 70%, while a dropout due tumor progress is reported in 23% (179,180).
In case of planed extended left trisectionectomy, a left PVE could be also considered (181).
ALPPS
ALPPS is a two-staged surgical procedure that consider ligation or transection of right PV (RPV) branch combined with parenchymal transection along the falciform ligament (182,183). The parenchymal transection avoids formation of new contralateral vessel as well as increases inflammation response. This technique has shown a 74% increase in the volume of the FRL in less than two weeks and could be also proposed as salvage after failed PVE (184).
However, it is also associated with high postoperative morbidity and mortality of quite 48% in patients with phCCA (185,186). For this reason, actually ALPPS is not recommended in patients with phCCA and should be indicated just in highly selected patients.
Preoperative biliary drainage (PBD)
Aim of the biliary drainage of the FRL is ameliorating the bile flow, primarily to relief the jaundice and improve liver function and secondarily to enhance the regeneration capacity of the liver, which may decrease PHLF risk and mortality. On the other hand, it increases infection rates and could seed tumor along the percutaneous catheter tract. This can result in sepsis and delay in therapy. For this reason, the use of PBD it still widely debated and studies demonstrating unconditional efficacy are lacking (54,187,188). A large European multicentric study showed reduced mortality in patients undergoing extended right hepatectomy, but curiously not in extended left (189), while an Asian meta-analysis did not show any benefit in patient with potentially resectable Klatskin tumor receiving a PBD (190).
Nowadays the use of a biliary stent is indicated in patients with congestive cholangitis, severe hyperbilirubinaemia-induced malnutrition or hepatic or renal insufficiency, as well as in patients undergoing preoperative PVE or neoadjuvant therapy (39,191). In patients with adequate nutritional status, slightly elevated bilirubin and no cholangitis the PBD could be avoided to prioritize surgery (190).
Some centers suggest a preoperative total bilirubin level of <2–3 mg/dL, and therefore PBD can still be recommended (39,188).
In case of bilateral cholestasis, the PBD should be placed on the FRL site to improve liver recovery and regeneration (69).
A PBD could be placed endoscopically by mean of a retrograde cholangiopancreatography [endoscopic biliary drainage (EBD) or nasobiliary]or percutaneous by mean of a transhepatic cholangiography (PTCD) (39,192). The main advantage of EBD is the reduce risk of seeding metastasis compared with PTCD. However, in patients undergoing PVE or chemotherapy it should be regularly changed. It can trigger ascending cholangitis in the FRL but it is not possible to sample the bile to obtain information about microbiology. An alternative could be the endoscopic nasobiliary drainage, which correlates with decreased cholangitis compared with EBD and permits sampling. Some groups recommend it as the ideal method. However, it is associated with patient discomfort imposed by nasal drainage (193,194). Furthermore, the time from EBD insertion to a satisfactory biliary level is faster with the endoscopic approach than with PTCD (33).
PTCD does not have to be regularly changed and permits sampling, too. However, due the transhepatic insertion it takes with it the risk of implantation metastasis as well as injury of the FRL.
In addition, PTCD has also a diagnostic role as they provide much better delineation of the intrahepatic extent of endobiliary tumour (195-197).
In addition, more than 50% of patients with an EBD require later a PTCD to achieve the required therapeutic effect (198).
In conclusion a PBD should be used just in case of strictly indication and possibly after the imaging is over to avoid artefacts.
Surgical pitfalls
Kind of resection
The decision of which kind of resection may allow the best radicality usually depends from the combination of the following factors:
- Tumor stage according to Bismuth-Corlette classification:
- IIIa: right trisectionectomy (ERH);
- IIIb: left hepatectomy (LH)—left trisectionectomy;
- IV: right/left trisectionectomy.
- FRL: in case of inadequate FRL and inability of augmentation of FRL, or in case of irreversible hypotrophy of one liver lobe, the strategy can be changed;
- Vascular infiltration: although nowadays complex vascular resection and reconstruction are feasible (but usually in tertiary centre with high experience in this context) the vascular infiltration of right or left vascular pedicle may influence the decision-making process of which kind of resection.
We suggest to perform whenever possible an ERH based on following aspects (12):
- Separate left hepatic vein providing independent venous drainage of segment 2 and 3;
- Umbilical plate (“own hilum”) containing separate BD and PV confluence (Rex sinus);
- Left hepatic duct (LHD) of long extrahepatic segment well accessible for surgical assessment.
Approach to the BD
For a correct approach to BD, following relevant anatomical aspects should be kept in mind (22):
The right hepatic duct (RHD) is more often involved by tumour up to its second-order branches because it is short and bifurcates early. The anatomy of the RHD bifurcation plays an important role in determining whether the right anterior sector is best preserved or resected. The RHD bifurcation has two major anatomical variations. The “normal” supraportal course of the right posterior sectoral duct (RPSD) is seen in approximately 85% of patients while the remaining have an infraportal RPSD (44,199,200). This anatomy is clearly discernable on cholangiography where the supraportal RPSD forms the so-called Hjortso’s Crook (44) A supraportal RPSD limits the extent of resection beyond the bifurcation of the RHD, is technically more difficult to anastomose and more likely to be associated with anastomotic leakage (15% vs. 0%) when the right anterior sector is preserved (199). Since the supraportal RPSD encircles the right anterior PV, resection of this vein and segments V and VIII permits resection of an additional 6–9 mm of BD and improves the likelihood of a negative surgical margin (89.6% vs. 97.7%) (44,200). On the other hand, patients with an infraportal RPSD are able to achieve a microscopically clear proximal surgical margin with parenchymal division just to the right of the principal plane (0% vs. 37% R1 resection) (199).
On the other side, the LHD is long and branches into its second-order ducts are far away from the hilum in the umbilical fissure.
The limit of BD resection towards the left is the left border of the umbilical portion of the LPV. This limit lies proximal to the confluence of B2 and B3 by about 5–10 mm and is reached after division of the portal branches to segment 4 (201).
The caudate lobe BDs drain close to the hilum and are invariably involved by tumour, which is why caudate lobe resection is an inherent part of the operation for phCCA. They travel superior to the PV bifurcation and this relationship with the PV explains why the superior aspect of the PV bifurcation is first to be involved by tumour (202,203).
According to the anatomical pillars mentioned above, the surgical approach to different stages of phCCA can vary as follows:
Bismuth-Corlette IIIa:
Two main options are available: (I) central resection (unusual) or (II) right trisectionectomy (ERH) (usual).
In case of infraportal anatomy of RHD (seldom) the tumour may be adequately resected with parenchymal resection of segments I and IVb. In case of supraportal anatomy of RHD (often) a central resection of segments I, IVb, V and VIII is needed.
Usually, when the left lateral section is of adequate volume (primary or after PVE) an ERH is an easier alternative to central resection. However, atrophy of the right lobe and/or involvement of right hepatic artery would take away the option of preserving the right posterior sector and make ERH necessary.
Bismuth-Corlette IIIb:
In case of absence of right lobar atrophy or vascular involvement a LH usually extended to the second order branches is the standard procedure.
Bismuth-Corlette IV:
In this case an ERH or partially extended LH are the main options. The choice between these operations is once again dictated by the nature of associated vascular involvement and parenchymal atrophy. If neither are present, a left-sided resection would be preferable in order to preserve parenchyma.
When the right lobe is atrophic or there is significant RPV involvement, the resection of choice is ERH. The left BD needs to be divided at the left border of the umbilical portion of the PV in order to extend the resection beyond the confluence of segments 2 and 3 BDs (201).
Approach to PV
phCCA often but not always do adhere or infiltrate the PV bifurcation. In the past such a situation was considered a sign of unresectability (25).
Nowadays, PV resection is now performed in 10–40% of phCCA resections with consequent increase of long-term survival rates but at costs of significant high rates of morbidity and mortality (23,25,204).
Therefore, it is actual consensus that PV bifurcation should be resected only when tumour adherence or tumor infiltration has been detected. In this context, the strategy of PV resection a priori according to the Neuhaus’ School has not been yet validated (22,24,205-208).
In case of right sided resection, the reconstruction of PV between the main stump and the left branch of PV in an end to end fashion is usually not a problem being due to its long extrahepatic length and easy access to the vein within the umbilical fissure beyond the limit of the tumour. In case of resection of more than 5-cm vein an interposition graft may be indicated.
The reconstruction of RPV is more demanding since the RPV is usually short and bifurcates early. In difficult cases a Y-interposition-graft may be necessary (22)
To avoid a twist of the PV anastomosis we suggest to put orientation stiches at the left side of both PV stumps before cutting the PV.
Approach to HA
Also, in this case following anatomical aspects should be taken in mind:
- The left HA (LHA) lies at the left border of the porta hepatis, away from the biliary confluence, travels straight to the Rex recess and is rarely involved by tumour. Notwithstanding, it is important to exclude involvement of the LHA within the umbilical fissure before embarking on ERH;
- The RHA runs between the BD anteriorly and the PV posteriorly, slightly inferior to the biliary confluence and because of its position is often involved in phCCA. It must be noted that contralateral involvement of the RHA is common even in left-sided phCCAs (type IIIb) and in such a case an arterial resection may become necessary. In this context a replaced RHA from the superior mesenteric artery may be advantageous since it lies to the right side of the biliary confluence so it may escape involvement in left sided tumours.
Arterial resection
The infiltration or abutment/encasement of the HA does not represent nowadays a contraindication to resection but it requires its resection and reconstruction. This is mainly true in case of left-sided resections (usually for phCCA Bismuth type IIIb) in which the RHA is wedged between tumour and PV and its maintenance is relevant for the arterial perfusion of the future remnant right liver lobe.
Resection with reconstruction with/without different interpositions grafts is technically demanding and requires high expertise (209). If arterial reconstruction can be correctly and safely performed the oncological results are excellent (i.e., 1-, 3- and 5-year survivals in this group of patients was 78.9%, 36.3% and 30.3%, respectively (210). Therefore, the reported results in the literature are quite different in terms of patency, morbidity and mortality rates (211-215).
In this context HBP tertiary centres with experience in segmental transplantation and microsurgical vascular reconstruction [i.e., living donor liver transplantation (LDLT) and pediatric liver transplantation) do offer the best results (22,210).
It should be noted that in same cases of left sided resections with involvement of RHA, the RHA itself can be excised a priori with no need of reconstruction at the only condition that mobilization of the right lobe of the liver has been kept to a minimum so as to preserve arterial collaterals from the diaphragm, intercostal vessels and retroperitoneal vessels (216-218). An alternative to that (i.e., impossibility of preservation of RHA and complete mobilisation of right liver lobe) the arterialization of PV can be considered as a salvage procedure (219).
Resection of caudate lobe (segment 1)
In all cases of phCCA Bismuth-Corlette III–IV the resection of segment 1 associated to right or left trisectionectomy is mandatory since the caudate lobe BDs drain close to the hilum and are invariably involved by tumour (22-24,192,220).
In this context, improved R0 resection rates and survival associated with caudate lobectomy for patients with Bismuth-Corlette type III and IV lesions have been demonstrated in several retrospective series (221-223).
Lymphadenectomy
Lymphnode involvement plays a significant negative prognostic value. Unfortunately, there is a poor correlation between lymph node size and positivity on imaging (224,225) being PET-scan eventually more specific (226).
Although the benefit of lymphadenectomy and its extent on patient survival remains controversial, the procedure is essential to obtain an accurate and reliable tumor staging (25,227).
Therefore, a systematic mandatory N1 lymphadenectomy (216-218) has been suggested by most of the authors (23,192) with possible but discussable extension to N2 level.
Hepatopancreatoduodenectomy
Hepatopancreatoduodenectomy is indicated for advanced phCCA with either distal biliary tract involvement or extensive lymph node metastases along the hepatoduodenal ligament and behind the pancreatic head to achieve surgical R0 resection (228,229).
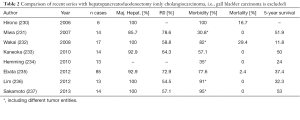
Recently published studies (Table 2) showed that this extended surgical approach in the context of local advanced CCA is feasible in highly specialized centers. Notwithstanding, due to the overall limited number of patients accompanied by high morbidity and also high mortality in some case series hepatopancreatoduodenectomy still remains controversial and a general recommendation cannot be given (238).
Full table
Perineural infiltration
Several retrospective studies including a recent meta-analysis (239) have identified perineural sheath invasion as an independent prognostic factor in terms of overall survival (23,31,204,206,227,240-254) as well as disease-free survival (255-257). Furthermore, its occurrence seems to be associated with a more advanced tumor stage particularly T-stage and UICC-stage (258,259). Despite these findings, perineural sheath infiltration, as well as other tumor-specific, non-surgical factors (e.g., grading, microvascular invasion), is yet not included in common classifications for phCCA aimed at prognosis (5,13,21,239,260,261). In this regard, a revised classification system might provide improved information for the prediction of patient survival (253) and potential risk of recurrence. This data could help to identify patients who possibly benefit most from adjuvant chemotherapy. Several partly ongoing trials investigate the value of adjuvant chemotherapy in the context of biliary tract cancers, but complete results of these trial are yet to be published (262-264).
In general, perineural sheath invasion has no influence on the extent of surgery, because its status only can be determined by histopathological assessment after completion of tumor resection.
Minimal invasive surgery
Due to its complexity phCCA still represents a purely open surgery procedure. The minimal invasive laparoscopic surgery does play role only as staging procedure (49,56).
Neoadjuvant treatment
Neoadjuvant treatment (radiotherapy, chemotherapy or combination of both procedures) for locally advanced phCCA seems not to influence the oncological outcome in terms of disease-free survival (DFS) and overall survival (OS). However, it may allow tumor downstaging and improve tumor resectability (49,265). Prospective randomized studies at this regard are still missing.
Conclusions
Surgery remains the only curative treatment of phCCA if a R0 situation can be reached.
For this reason, advanced phCCA usually requires an extended hepatic resection and often a vascular resection.
In order to reach a R0 situation and to avoid a PHLF an accurate preoperative interdisciplinary study of the tumor extension, liver status (i.e., volume, quality and function of FRL) is mandatory.
A predominantly right-sided tumour is best treated by extended right hepatectomy and is likely to need PV resection.
An advanced left-sided tumour requires left trisectionectomy preferably using a left-sided approach to resection, and is likely to require PV as well as right hepatic arterial resection.
These are technically challenging operations and must be performed in a high volume centres by surgeons with experience in microsurgical techniques.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Altemeier WA, Gall EA, Zinninger MM, et al. Sclerosing carcinoma of the major intrahepatic bile ducts. AMA Arch Surg 1957;75:450-60; discussion 460-1. [Crossref] [PubMed]
- Klatskin G. Adenocarcinoma of the Hepatic Duct at Its Bifurcation within the Porta Hepatis. An Unusual Tumor with Distinctive Clinical and Pathological Features. Am J Med 1965;38:241-56. [Crossref] [PubMed]
- Nagorney DM, Pawlik TM, Chun YS, et al. In: Amin MB. editor. AJCC Cancer Staging Manual. 8th edition. Chicago: AJCC, 2017:311.
- Japanese Society of Biliary Surgery. General rules for surgical and pathological study on cancer of the biliary tract. 4th edition. Tokyo: Kanehara, 1997.
- Ebata T, Kosuge T, Hirano S, et al. Proposal to modify the International Union Against Cancer staging system for perihilar cholangiocarcinomas. Br J Surg 2014;101:79-88. [Crossref] [PubMed]
- DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755-62. [Crossref] [PubMed]
- Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol 2012;56:848-54. [Crossref] [PubMed]
- Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261-80. [Crossref] [PubMed]
- Ghouri YA, Mian I, Blechacz B. Cancer review: Cholangiocarcinoma. J Carcinog 2015;14:1. [Crossref] [PubMed]
- Saha SK, Zhu AX, Fuchs CS, et al. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016;21:594-9. [Crossref] [PubMed]
- Cidon EU. Resectable Cholangiocarcinoma: Reviewing the Role of Adjuvant Strategies. Clin Med Insights Oncol 2016;10:43-8. [Crossref] [PubMed]
- Radtke A, Konigsrainer A. Surgical Therapy of Cholangiocarcinoma. Visc Med 2016;32:422-6. [Crossref] [PubMed]
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517-9. [Crossref] [PubMed]
- Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 1975;140:170-8. [PubMed]
- Hemming AW, Reed AI, Fujita S, et al. Surgical management of hilar cholangiocarcinoma. Ann Surg 2005;241:693-9; discussion 699-702. [Crossref] [PubMed]
- Juntermanns B, Sotiropoulos GC, Radunz S, et al. Comparison of the sixth and the seventh editions of the UICC classification for perihilar cholangiocarcinoma. Ann Surg Oncol 2013;20:277-84.
- Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 2012;215:343-55. [Crossref] [PubMed]
- Blechacz BR, Sanchez W, Gores GJ. A conceptual proposal for staging ductal cholangiocarcinoma. Curr Opin Gastroenterol 2009;25:238-9. [Crossref] [PubMed]
- Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology 2011;53:1363-71. [Crossref] [PubMed]
- Govil S, Reddy MS, Rela M. Surgical resection techniques for locally advanced hilar cholangiocarcinoma. Langenbecks Arch Surg 2014;399:707-16. [Crossref] [PubMed]
- Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013;258:129-40. [Crossref] [PubMed]
- Nagino M. Perihilar cholangiocarcinoma: a surgeon's viewpoint on current topics. J Gastroenterol 2012;47:1165-76. [Crossref] [PubMed]
- Ramos E. Principles of surgical resection in hilar cholangiocarcinoma. World J Gastrointest Oncol 2013;5:139-46. [Crossref] [PubMed]
- Valero V 3rd, Cosgrove D, Herman JM, et al. Management of perihilar cholangiocarcinoma in the era of multimodal therapy. Expert Rev Gastroenterol Hepatol 2012;6:481-95. [Crossref] [PubMed]
- LaFemina J, Jarnagin WR. Surgical management of proximal bile duct cancers. Langenbecks Arch Surg 2012;397:869-79. [Crossref] [PubMed]
- Miyazaki M, Kimura F, Shimizu H, et al. One hundred seven consecutive surgical resections for hilar cholangiocarcinoma of Bismuth types II, III, IV between 2001 and 2008. J Hepatobiliary Pancreat Sci 2010;17:470-5. [Crossref] [PubMed]
- Launois B, Reding R, Lebeau G, et al. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg 2000;7:128-34. [Crossref] [PubMed]
- Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg 2003;238:73-83. [Crossref] [PubMed]
- Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg 2012;147:26-34. [Crossref] [PubMed]
- Hasegawa S, Ikai I, Fujii H, et al. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg 2007;31:1256-63. [Crossref] [PubMed]
- Bhardwaj N, Garcea G, Dennison AR, et al. The Surgical Management of Klatskin Tumours: Has Anything Changed in the Last Decade? World J Surg 2015;39:2748-56. [Crossref] [PubMed]
- Aloia TA, Charnsangavej C, Faria S, et al. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am J Surg 2007;193:702-6. [Crossref] [PubMed]
- Lee HY, Kim SH, Lee JM, et al. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology 2006;239:113-21. [Crossref] [PubMed]
- Unno M, Okumoto T, Katayose Y, et al. Preoperative assessment of hilar cholangiocarcinoma by multidetector row computed tomography. J Hepatobiliary Pancreat Surg 2007;14:434-40. [Crossref] [PubMed]
- Vogl TJ, Schwarz WO, Heller M, et al. Staging of Klatskin tumours (hilar cholangiocarcinomas): comparison of MR cholangiography, MR imaging, and endoscopic retrograde cholangiography. Eur Radiol 2006;16:2317-25. [Crossref] [PubMed]
- Mangold S, Bretschneider C, Fenchel M, et al. MRI for evaluation of potential living liver donors: a new approach including contrast-enhanced magnetic resonance cholangiography. Abdom Imaging 2012;37:244-51. [Crossref] [PubMed]
- Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:691-9. [Crossref] [PubMed]
- Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 2010;257:674-84. [Crossref] [PubMed]
- Masselli G, Gualdi G. Hilar cholangiocarcinoma: MRI/MRCP in staging and treatment planning. Abdom Imaging 2008;33:444-51. [Crossref] [PubMed]
- Moon CM, Bang S, Chung JB. The role of (18)F-fluorodeoxyglucose positron emission tomography in the diagnosis, staging, and follow-up of cholangiocarcinoma. Surg Oncol 2011;20:e10-7. [Crossref] [PubMed]
- Ruys AT, Bennink RJ, van Westreenen HL, et al. FDG-positron emission tomography/computed tomography and standardized uptake value in the primary diagnosis and staging of hilar cholangiocarcinoma. HPB (Oxford) 2011;13:256-62. [Crossref] [PubMed]
- Shimizu H, Sawada S, Kimura F, et al. Clinical significance of biliary vascular anatomy of the right liver for hilar cholangiocarcinoma applied to left hemihepatectomy. Ann Surg 2009;249:435-9. [Crossref] [PubMed]
- Xiong JJ, Nunes QM, Huang W, et al. Preoperative biliary drainage in patients with hilar cholangiocarcinoma undergoing major hepatectomy. World J Gastroenterol 2013;19:8731-9. [Crossref] [PubMed]
- Victor DW, Sherman S, Karakan T, et al. Current endoscopic approach to indeterminate biliary strictures. World J Gastroenterol 2012;18:6197-205. [Crossref] [PubMed]
- Hara K, Yamao K, Mizuno N, et al. Endoscopic ultrasonography-guided biliary drainage: Who, when, which, and how? World J Gastroenterol 2016;22:1297-303. [Crossref] [PubMed]
- De Bellis M, Sherman S, Fogel EL, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 1). Gastrointest Endosc 2002;56:552-61. [Crossref] [PubMed]
- Rassam F, Roos E, van Lienden KP, et al. Modern work-up and extended resection in perihilar cholangiocarcinoma: the AMC experience. Langenbecks Arch Surg 2018;403:289-307. [Crossref] [PubMed]
- Liu R, Cox Rn K, Siddiqui A, et al. Peroral cholangioscopy facilitates targeted tissue acquisition in patients with suspected cholangiocarcinoma. Minerva Gastroenterol Dietol 2014;60:127-33. [PubMed]
- Navaneethan U, Hasan MK, Lourdusamy V, et al. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc 2015;82:608-14.e2. [Crossref] [PubMed]
- Heimbach JK, Sanchez W, Rosen CB, et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356-60. [Crossref] [PubMed]
- Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006;243:364-72. [Crossref] [PubMed]
- Rocha FG, Matsuo K, Blumgart LH, et al. Hilar cholangiocarcinoma: the Memorial Sloan-Kettering Cancer Center experience. J Hepatobiliary Pancreat Sci 2010;17:490-6. [Crossref] [PubMed]
- Song SC, Choi DW, Kow AW, et al. Surgical outcomes of 230 resected hilar cholangiocarcinoma in a single centre. ANZ J Surg 2013;83:268-74. [Crossref] [PubMed]
- Bird N, Elmasry M, Jones R, et al. Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. Br J Surg 2017;104:418-25. [Crossref] [PubMed]
- Groot Koerkamp B, Wiggers JK, Gonen M, et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol 2016;27:753. [Crossref] [PubMed]
- Tilleman EH, de Castro SM, Busch OR, et al. Diagnostic laparoscopy and laparoscopic ultrasound for staging of patients with malignant proximal bile duct obstruction. J Gastrointest Surg 2002;6:426-30; discussion 430-1. [Crossref] [PubMed]
- Connor S, Barron E, Wigmore SJ, et al. The utility of laparoscopic assessment in the preoperative staging of suspected hilar cholangiocarcinoma. J Gastrointest Surg 2005;9:476-80. [Crossref] [PubMed]
- Goere D, Wagholikar GD, Pessaux P, et al. Utility of staging laparoscopy in subsets of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc 2006;20:721-5. [Crossref] [PubMed]
- Barlow AD, Garcea G, Berry DP, et al. Staging laparoscopy for hilar cholangiocarcinoma in 100 patients. Langenbecks Arch Surg 2013;398:983-8. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut 2002;51 Suppl 6:VI1-9. [Crossref] [PubMed]
- Eguchi H, Umeshita K, Sakon M, et al. Presence of active hepatitis associated with liver cirrhosis is a risk factor for mortality caused by posthepatectomy liver failure. Dig Dis Sci 2000;45:1383-8. [Crossref] [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406; discussion 406-7. [Crossref] [PubMed]
- Tzeng CW, Cooper AB, Vauthey JN, et al. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford) 2014;16:459-68. [Crossref] [PubMed]
- Golse N, Bucur PO, Adam R, et al. New paradigms in post-hepatectomy liver failure. J Gastrointest Surg 2013;17:593-605. [Crossref] [PubMed]
- Aloia TA, Fahy BN, Fischer CP, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford) 2009;11:510-5. [Crossref] [PubMed]
- Wiggers JK, Groot Koerkamp B, Cieslak KP, et al. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J Am Coll Surg 2016;223:321-331.e1. [Crossref] [PubMed]
- Rahnemai-Azar AA, Cloyd JM, Weber SM, et al. Update on Liver Failure Following Hepatic Resection: Strategies for Prediction and Avoidance of Post-operative Liver Insufficiency. J Clin Transl Hepatol 2018;6:97-104. [Crossref] [PubMed]
- Valero V 3rd, Amini N, Spolverato G, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg 2015;19:272-81. [Crossref] [PubMed]
- Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation 2006;82:1703-7. [Crossref] [PubMed]
- Park J, Kim MH, Kim KP, et al. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver 2009;3:298-305. [Crossref] [PubMed]
- Olthof PB, Wiggers JK, Groot Koerkamp B, et al. Postoperative Liver Failure Risk Score: Identifying Patients with Resectable Perihilar Cholangiocarcinoma Who Can Benefit from Portal Vein Embolization. J Am Coll Surg 2017;225:387-94. [Crossref] [PubMed]
- Shoup M, Gonen M, D'Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 2003;7:325-30. [Crossref] [PubMed]
- Truant S, Oberlin O, Sergent G, et al. Remnant liver volume to body weight ratio > or =0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg 2007;204:22-33. [Crossref] [PubMed]
- Lo CM, Fan ST, Chan JK, et al. Minimum graft volume for successful adult-to-adult living donor liver transplantation for fulminant hepatic failure. Transplantation 1996;62:696-8. [Crossref] [PubMed]
- Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol 2008;25:104-9. [Crossref] [PubMed]
- Nadalin S, Capobianco I, Li J, et al. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons Learned from 15 cases at a single centre. Z Gastroenterol 2014;52:35-42. [Crossref] [PubMed]
- Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg 2009;197:686-90. [Crossref] [PubMed]
- Helling TS. Liver failure following partial hepatectomy. HPB (Oxford) 2006;8:165-74. [Crossref] [PubMed]
- Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1271-80. [Crossref] [PubMed]
- Zorzi D, Laurent A, Pawlik TM, et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg 2007;94:274-86. [Crossref] [PubMed]
- Alizai PH, Haelsig A, Bruners P, et al. Impact of liver volume and liver function on posthepatectomy liver failure after portal vein embolization- A multivariable cohort analysis. Ann Med Surg (Lond) 2017;25:6-11. [Crossref] [PubMed]
- Schreckenbach T, Liese J, Bechstein WO, et al. Posthepatectomy liver failure. Dig Surg 2012;29:79-85. [Crossref] [PubMed]
- Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008;13:51-64. [Crossref] [PubMed]
- Pomposelli JJ, Tongyoo A, Wald C, et al. Variability of standard liver volume estimation versus software-assisted total liver volume measurement. Liver Transpl 2012;18:1083-92. [Crossref] [PubMed]
- Soyer P, Roche A, Elias D, et al. Hepatic metastases from colorectal cancer: influence of hepatic volumetric analysis on surgical decision making. Radiology 1992;184:695-7. [Crossref] [PubMed]
- Suzuki K, Epstein ML, Kohlbrenner R, et al. Quantitative radiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. AJR Am J Roentgenol 2011;197. [Crossref] [PubMed]
- D'Onofrio M, De Robertis R, Demozzi E, et al. Liver volumetry: Is imaging reliable? Personal experience and review of the literature. World J Radiol 2014;6:62-71. [Crossref] [PubMed]
- Tu R, Xia LP, Yu AL, et al. Assessment of hepatic functional reserve by cirrhosis grading and liver volume measurement using CT. World J Gastroenterol 2007;13:3956-61. [Crossref] [PubMed]
- Torzilli G, Montorsi M, Del Fabbro D, et al. Ultrasonographically guided surgical approach to liver tumours involving the hepatic veins close to the caval confluence. Br J Surg 2006;93:1238-46. [Crossref] [PubMed]
- Ulla M, Ardiles V, Levy-Yeyati E, et al. New surgical strategy to induce liver hypertrophy: role of MDCT-volumetry to monitor and predict liver growth. Hepatogastroenterology 2013;60:337-42. [PubMed]
- Ercolani G, Zanello M, Grazi GL, et al. Changes in the surgical approach to hilar cholangiocarcinoma during an 18-year period in a Western single center. J Hepatobiliary Pancreat Sci 2010;17:329-37. [Crossref] [PubMed]
- Sotiropoulos GC, Saner FH, Molmenti EP, et al. Unexpected liver failure after right hemihepatectomy for colorectal liver metastasis due to chemotherapy-associated steato-hepatitis: time for routine preoperative liver biopsy? Int J Colorectal Dis 2009;24:241. [Crossref] [PubMed]
- Cucchetti A, Cescon M, Ercolani G, et al. Safety of hepatic resection in overweight and obese patients with cirrhosis. Br J Surg 2011;98:1147-54. [Crossref] [PubMed]
- Vetelainen R, van Vliet A, Gouma DJ, et al. Steatosis as a risk factor in liver surgery. Ann Surg 2007;245:20-30. [Crossref] [PubMed]
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996;23:1079-83. [Crossref] [PubMed]
- McGill DB, Rakela J, Zinsmeister AR, et al. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology 1990;99:1396-400. [Crossref] [PubMed]
- Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898-906. [Crossref] [PubMed]
- Fujiwara Y, Kuroda H, Abe T, et al. The B-Mode Image-Guided Ultrasound Attenuation Parameter Accurately Detects Hepatic Steatosis in Chronic Liver Disease. Ultrasound Med Biol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Allan R, Thoirs K, Phillips M. Accuracy of ultrasound to identify chronic liver disease. World J Gastroenterol 2010;16:3510-20. [Crossref] [PubMed]
- Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med 2013;34:238-53. [Crossref] [PubMed]
- Han H, Hu H, Xu YD, et al. Liver failure after hepatectomy: A risk assessment using the pre-hepatectomy shear wave elastography technique. Eur J Radiol 2017;86:234-40. [Crossref] [PubMed]
- Lei JW, Ji XY, Hong JF, et al. Prediction of posthepatectomy liver failure using transient elastography in patients with hepatitis B related hepatocellular carcinoma. BMC Gastroenterol 2017;17:171. [Crossref] [PubMed]
- Nishio T, Taura K, Koyama Y, et al. Prediction of posthepatectomy liver failure based on liver stiffness measurement in patients with hepatocellular carcinoma. Surgery 2016;159:399-408. [Crossref] [PubMed]
- Lee SS, Park SH, Kim HJ, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol 2010;52:579-85. [Crossref] [PubMed]
- McDonald N, Eddowes PJ, Hodson J, et al. Multiparametric magnetic resonance imaging for quantitation of liver disease: a two-centre cross-sectional observational study. Sci Rep 2018;8:9189. [Crossref] [PubMed]
- Imajo K, Kessoku T, Honda Y, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016;150:626-637.e7. [Crossref] [PubMed]
- Lv S, Jiang S, Liu S, et al. Noninvasive Quantitative Detection Methods of Liver Fat Content in Nonalcoholic Fatty Liver Disease. J Clin Transl Hepatol 2018;6:217-21. [Crossref] [PubMed]
- van Werven JR, Marsman HA, Nederveen AJ, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology 2010;256:159-68. [Crossref] [PubMed]
- Bohte AE, Garteiser P, De Niet A, et al. MR elastography of the liver: defining thresholds for detecting viscoelastic changes. Radiology 2013;269:768-76. [Crossref] [PubMed]
- Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging 2013;37:544-55. [Crossref] [PubMed]
- Panicek DM, Giess CS, Schwartz LH. Qualitative assessment of liver for fatty infiltration on contrast-enhanced CT: is muscle a better standard of reference than spleen? J Comput Assist Tomogr 1997;21:699-705. [Crossref] [PubMed]
- Donadon M, Cimino M, Procopio F, et al. Potential role of cholinesterases to predict short-term outcome after hepatic resection for hepatocellular carcinoma. Updates Surg 2013;65:11-8. [Crossref] [PubMed]
- Nadalin S, Testa G, Malago M, et al. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver Transpl 2004;10:1024-9. [Crossref] [PubMed]
- Nagashima I, Takada T, Okinaga K, et al. A scoring system for the assessment of the risk of mortality after partial hepatectomy in patients with chronic liver dysfunction. J Hepatobiliary Pancreat Surg 2005;12:44-8. [Crossref] [PubMed]
- Garcea G, Ong SL, Maddern GJ. Predicting liver failure following major hepatectomy. Dig Liver Dis 2009;41:798-806. [Crossref] [PubMed]
- Schroeder RA, Marroquin CE, Bute BP, et al. Predictive indices of morbidity and mortality after liver resection. Ann Surg 2006;243:373-9. [Crossref] [PubMed]
- Kamath PS, Kim WR. Advanced Liver Disease Study G. The model for end-stage liver disease (MELD). Hepatology 2007;45:797-805. [Crossref] [PubMed]
- Zheng Y, Yang H, He L, et al. Reassessment of different criteria for diagnosing post-hepatectomy liver failure: a single-center study of 1683 hepatectomy. Oncotarget 2017;8:89269-77. [PubMed]
- Cieslak KP, Runge JH, Heger M, et al. New perspectives in the assessment of future remnant liver. Dig Surg 2014;31:255-68. [Crossref] [PubMed]
- Caesar J, Shaldon S, Chiandussi L, et al. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci 1961;21:43-57. [PubMed]
- Paumgartner G. The handling of indocyanine green by the liver. Schweiz Med Wochenschr 1975;105:1-30. [PubMed]
- de Liguori Carino N, O'Reilly DA, Dajani K, et al. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur J Surg Oncol 2009;35:957-62. [Crossref] [PubMed]
- Lau H, Man K, Fan ST, et al. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg 1997;84:1255-9. [Crossref] [PubMed]
- Fan ST, Lai EC, Lo CM, et al. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg 1995;130:198-203. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004;240:698-708; discussion 708-10. [PubMed]
- Imamura H, Sano K, Sugawara Y, et al. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg 2005;12:16-22. [Crossref] [PubMed]
- Fazakas J, Mandli T, Ther G, et al. Evaluation of liver function for hepatic resection. Transplant Proc 2006;38:798-800. [Crossref] [PubMed]
- Stockmann M, Lock JF, Riecke B, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg 2009;250:119-25. [Crossref] [PubMed]
- Stockmann M, Lock JF, Malinowski M, et al. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB (Oxford) 2010;12:139-46. [Crossref] [PubMed]
- Hoekstra LT, de Graaf W, Nibourg GA, et al. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg 2013;257:27-36. [Crossref] [PubMed]
- Mimura T, Hamazaki K, Sakai H, et al. Evaluation of hepatic functional reserve in rats with obstructive jaundice by asyaloglycoprotein receptor. Hepatogastroenterology 2001;48:777-82. [PubMed]
- Satoh K, Yamamoto Y, Nishiyama Y, et al. 99mTc-GSA liver dynamic SPECT for the preoperative assessment of hepatectomy. Ann Nucl Med 2003;17:61-7. [Crossref] [PubMed]
- Beppu T, Hayashi H, Okabe H, et al. Liver functional volumetry for portal vein embolization using a newly developed 99mTc-galactosyl human serum albumin scintigraphy SPECT-computed tomography fusion system. J Gastroenterol 2011;46:938-43. [Crossref] [PubMed]
- Iimuro Y, Kashiwagi T, Yamanaka J, et al. Preoperative estimation of asialoglycoprotein receptor expression in the remnant liver from CT/99mTc-GSA SPECT fusion images correlates well with postoperative liver function parameters. J Hepatobiliary Pancreat Sci 2010;17:673-81. [Crossref] [PubMed]
- Kim YK, Nakano H, Yamaguchi M, et al. Prediction of postoperative decompensated liver function by technetium-99m galactosyl-human serum albumin liver scintigraphy in patients with hepatocellular carcinoma complicating chronic liver disease. Br J Surg 1997;84:793-6. [Crossref] [PubMed]
- Kokudo N, Vera DR, Tada K, et al. Predictors of successful hepatic resection: prognostic usefulness of hepatic asialoglycoprotein receptor analysis. World J Surg 2002;26:1342-7. [Crossref] [PubMed]
- Nanashima A, Yamaguchi H, Shibasaki S, et al. Relationship between indocyanine green test and technetium-99m galactosyl serum albumin scintigraphy in patients scheduled for hepatectomy: Clinical evaluation and patient outcome. Hepatol Res 2004;28:184-90. [Crossref] [PubMed]
- Takeuchi S, Nakano H, Kim YK, et al. Predicting survival and post-operative complications with Tc-GSA liver scintigraphy in hepatocellular carcinoma. Hepatogastroenterology 1999;46:1855-61. [PubMed]
- Hirai I, Kimura W, Fuse A, et al. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99mTc-GSA SPECT scintigraphy. Surgery 2003;133:495-506. [Crossref] [PubMed]
- Nishiyama Y, Yamamoto Y, Hino I, et al. 99mTc galactosyl human serum albumin liver dynamic SPET for pre-operative assessment of hepatectomy in relation to percutaneous transhepatic portal embolization. Nucl Med Commun 2003;24:809-17. [Crossref] [PubMed]
- de Graaf W, van Lienden KP, van Gulik TM, et al. (99m)Tc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J Nucl Med 2010;51:229-36. [Crossref] [PubMed]
- de Graaf W, Hausler S, Heger M, et al. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J Hepatol 2011;54:738-45. [Crossref] [PubMed]
- Krishnamurthy GT, Krishnamurthy S. Cholescintigraphic measurement of liver function: how is it different from other methods? Eur J Nucl Med Mol Imaging 2006;33:1103-6. [Crossref] [PubMed]
- Krishnamurthy S, Krishnamurthy GT. Technetium-99m-iminodiacetic acid organic anions: review of biokinetics and clinical application in hepatology. Hepatology 1989;9:139-53. [Crossref] [PubMed]
- Loberg MD, Cooper M, Harvey E, et al. Development of new radiopharmaceuticals based on N-substitution of iminodiacetic acid. J Nucl Med 1976;17:633-8. [PubMed]
- Erdogan D, Heijnen BH, Bennink RJ, et al. Preoperative assessment of liver function: a comparison of 99mTc-Mebrofenin scintigraphy with indocyanine green clearance test. Liver Int 2004;24:117-23. [Crossref] [PubMed]
- Bennink RJ, Dinant S, Erdogan D, et al. Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J Nucl Med 2004;45:965-71. [PubMed]
- de Graaf W, Bennink RJ, Vetelainen R, et al. Nuclear imaging techniques for the assessment of hepatic function in liver surgery and transplantation. J Nucl Med 2010;51:742-52. [Crossref] [PubMed]
- Dinant S, de Graaf W, Verwer BJ, et al. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med 2007;48:685-92. [Crossref] [PubMed]
- Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med 1998;339:1217-27. [Crossref] [PubMed]
- de Graaf W, van Lienden KP, Dinant S, et al. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg 2010;14:369-78. [Crossref] [PubMed]
- Schuhmann-Giampieri G. Liver contrast media for magnetic resonance imaging. Interrelations between pharmacokinetics and imaging. Invest Radiol 1993;28:753-61. [Crossref] [PubMed]
- Nilsson H, Blomqvist L, Douglas L, et al. Gd-EOB-DTPA-enhanced MRI for the assessment of liver function and volume in liver cirrhosis. Br J Radiol 2013;86. [Crossref] [PubMed]
- Tschirch FT, Struwe A, Petrowsky H, et al. Contrast-enhanced MR cholangiography with Gd-EOB-DTPA in patients with liver cirrhosis: visualization of the biliary ducts in comparison with patients with normal liver parenchyma. Eur Radiol 2008;18:1577-86. [Crossref] [PubMed]
- Motosugi U, Ichikawa T, Sou H, et al. Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement? J Magn Reson Imaging 2009;30:1042-6. [Crossref] [PubMed]
- Takao H, Akai H, Tajima T, et al. MR imaging of the biliary tract with Gd-EOB-DTPA: effect of liver function on signal intensity. Eur J Radiol 2011;77:325-9. [Crossref] [PubMed]
- Tajima T. The relationship between self-concept variability and psychological well-being: a survey of female undergraduate students. Shinrigaku Kenkyu 2010;81:523-8. [Crossref] [PubMed]
- Katsube T, Okada M, Kumano S, et al. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Invest Radiol 2011;46:277-83. [Crossref] [PubMed]
- Nishie A, Ushijima Y, Tajima T, et al. Quantitative analysis of liver function using superparamagnetic iron oxide- and Gd-EOB-DTPA-enhanced MRI: comparison with Technetium-99m galactosyl serum albumin scintigraphy. Eur J Radiol 2012;81:1100-4. [Crossref] [PubMed]
- Nilsson H, Blomqvist L, Douglas L, et al. Assessment of liver function in primary biliary cirrhosis using Gd-EOB-DTPA-enhanced liver MRI. HPB (Oxford) 2010;12:567-76. [Crossref] [PubMed]
- Radtke A, Sgourakis G, Molmenti EP, et al. Computer-assisted surgical planning in adult-to-adult live donor liver transplantation: how much does it help? A single center experience. Transplantation 2012;94:1138-44. [Crossref] [PubMed]
- Radtke A, Sotiropoulos GC, Molmenti EP, et al. Computer-assisted surgery planning for complex liver resections: when is it helpful? A single-center experience over an 8-year period. Ann Surg 2010;252:876-83. [Crossref] [PubMed]
- Radtke A, Sgourakis G, Sotiropoulos GC, et al. Hepatic hilar and sectorial vascular and biliary anatomy in right graft adult live liver donor transplantation. Transplant Proc 2008;40:3147-50. [Crossref] [PubMed]
- Lhuaire M, Piardi T, Bruno O, et al. Post-hepatectomy liver failure: Should we consider venous outflow? Int J Surg Case Rep 2015;16:154-6. [Crossref] [PubMed]
- Dirsch O, Madrahimov N, Chaudri N, et al. Recovery of liver perfusion after focal outflow obstruction and liver resection. Transplantation 2008;85:748-56. [Crossref] [PubMed]
- Radtke A, Nadalin S, Sotiropoulos GC, et al. Computer-assisted operative planning in adult living donor liver transplantation: a new way to resolve the dilemma of the middle hepatic vein. World J Surg 2007;31:175-85. [Crossref] [PubMed]
- Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521-7. [PubMed]
- Thakrar PD, Madoff DC. Preoperative portal vein embolization: an approach to improve the safety of major hepatic resection. Semin Roentgenol 2011;46:142-53. [Crossref] [PubMed]
- Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49-57. [Crossref] [PubMed]
- Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386-94. [Crossref] [PubMed]
- Shindoh J, Tzeng CW, Aloia TA, et al. Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J Gastrointest Surg 2014;18:45-51. [Crossref] [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [Crossref] [PubMed]
- Kishi Y, Madoff DC, Abdalla EK, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery 2008;144:744-51. [Crossref] [PubMed]
- Hwang S, Ha TY, Ko GY, et al. Preoperative Sequential Portal and Hepatic Vein Embolization in Patients with Hepatobiliary Malignancy. World J Surg 2015;39:2990-8. [Crossref] [PubMed]
- Munene G, Parker RD, Larrigan J, et al. Sequential preoperative hepatic vein embolization after portal vein embolization for extended left hepatectomy in colorectal liver metastases. World J Surg Oncol 2013;11:134. [Crossref] [PubMed]
- Yamashita S, Sakamoto Y, Yamamoto S, et al. Efficacy of Preoperative Portal Vein Embolization Among Patients with Hepatocellular Carcinoma, Biliary Tract Cancer, and Colorectal Liver Metastases: A Comparative Study Based on Single-Center Experience of 319 Cases. Ann Surg Oncol 2017;24:1557-68. [Crossref] [PubMed]
- Sun Z, Tang W, Sakamoto Y, et al. A systematic review and meta-analysis of feasibility, safety and efficacy of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH). Biosci Trends 2015;9:284-8. [Crossref] [PubMed]
- Uesaka K. Left hepatectomy or left trisectionectomy with resection of the caudate lobe and extrahepatic bile duct for hilar cholangiocarcinoma (with video). J Hepatobiliary Pancreat Sci 2012;19:195-202. [Crossref] [PubMed]
- Li J, Girotti P, Konigsrainer I, et al. ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg 2013;17:956-61. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Enne M, Schadde E, Bjornsson B, et al. ALPPS as a salvage procedure after insufficient future liver remnant hypertrophy following portal vein occlusion. HPB (Oxford) 2017;19:1126-9. [Crossref] [PubMed]
- Eshmuminov D, Raptis DA, Linecker M, et al. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg 2016;103:1768-82. [Crossref] [PubMed]
- Olthof PB, Coelen RJS, Wiggers JK, et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford) 2017;19:381-7. [Crossref] [PubMed]
- Iacono C, Ruzzenente A, Campagnaro T, et al. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg 2013;257:191-204. [Crossref] [PubMed]
- Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro). HPB (Oxford) 2008;10:130-3. [Crossref] [PubMed]
- Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg 2013;100:274-83. [Crossref] [PubMed]
- Liu F, Li Y, Wei Y, et al. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci 2011;56:663-72. [Crossref] [PubMed]
- Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9:43-57. [Crossref] [PubMed]
- Nimura Y, Kamiya J, Kondo S, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg 2000;7:155-62. [Crossref] [PubMed]
- Kawakami H, Kuwatani M, Onodera M, et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol 2011;46:242-8. [Crossref] [PubMed]
- Kawashima H, Itoh A, Ohno E, et al. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: a retrospective study of efficacy and risk factors related to complications. Ann Surg 2013;257:121-7. [Crossref] [PubMed]
- van Delden OM, Lameris JS. Percutaneous drainage and stenting for palliation of malignant bile duct obstruction. Eur Radiol 2008;18:448-56. [Crossref] [PubMed]
- George C, Byass OR, Cast JE. Interventional radiology in the management of malignant biliary obstruction. World J Gastrointest Oncol 2010;2:146-50. [Crossref] [PubMed]
- Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc 2009;69:55-62. [Crossref] [PubMed]
- Walter T, Ho CS, Horgan AM, et al. Endoscopic or percutaneous biliary drainage for Klatskin tumors? J Vasc Interv Radiol 2013;24:113-21. [Crossref] [PubMed]
- Hirano S, Tanaka E, Shichinohe T, et al. Treatment strategy for hilar cholangiocarcinoma, with special reference to the limits of ductal resection in right-sided hepatectomies. J Hepatobiliary Pancreat Surg 2007;14:429-33. [Crossref] [PubMed]
- Natsume S, Ebata T, Yokoyama Y, et al. Clinical significance of left trisectionectomy for perihilar cholangiocarcinoma: an appraisal and comparison with left hepatectomy. Ann Surg 2012;255:754-62. [Crossref] [PubMed]
- Hosokawa I, Shimizu H, Yoshidome H, et al. Surgical strategy for hilar cholangiocarcinoma of the left-side predominance: current role of left trisectionectomy. Ann Surg 2014;259:1178-85. [Crossref] [PubMed]
- Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl 2011;17:1127-36. [Crossref] [PubMed]
- Reichert PR, Renz JF, D'Albuquerque LA, et al. Surgical anatomy of the left lateral segment as applied to living-donor and split-liver transplantation: a clinicopathologic study. Ann Surg 2000;232:658-64. [Crossref] [PubMed]
- de Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer 2012;118:4737-47. [Crossref] [PubMed]
- Hirano S, Kondo S, Tanaka E, et al. No-touch resection of hilar malignancies with right hepatectomy and routine portal reconstruction. J Hepatobiliary Pancreat Surg 2009;16:502-7. [Crossref] [PubMed]
- Neuhaus P, Thelen A, Jonas S, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol 2012;19:1602-8. [Crossref] [PubMed]
- Tamoto E, Hirano S, Tsuchikawa T, et al. Portal vein resection using the no-touch technique with a hepatectomy for hilar cholangiocarcinoma. HPB (Oxford) 2014;16:56-61. [Crossref] [PubMed]
- Rela M, Rajalingam R, Shanmugam V, et al. Novel en-bloc resection of locally advanced hilar cholangiocarcinoma: the Rex recess approach. Hepatobiliary Pancreat Dis Int 2014;13:93-7. [Crossref] [PubMed]
- Uchiyama H, Shirabe K, Araki K, et al. Left hepatectomy with simultaneous hepatic artery and portal vein reconstructions in the operation for cholangiocarcinoma: the surgical techniques comprised of step-by-step established procedures. Transl Gastroenterol Hepatol 2017;2:34. [Crossref] [PubMed]
- Nagino M, Nimura Y, Nishio H, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg 2010;252:115-23. [Crossref] [PubMed]
- Miyazaki M, Kato A, Ito H, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery 2007;141:581-8. [Crossref] [PubMed]
- Sakamoto Y, Sano T, Shimada K, et al. Clinical significance of reconstruction of the right hepatic artery for biliary malignancy. Langenbecks Arch Surg 2006;391:203-8. [Crossref] [PubMed]
- Shimada H, Endo I, Sugita M, et al. Hepatic resection combined with portal vein or hepatic artery reconstruction for advanced carcinoma of the hilar bile duct and gallbladder. World J Surg 2003;27:1137-42. [Crossref] [PubMed]
- Yamanaka N, Yasui C, Yamanaka J, et al. Left hemihepatectomy with microsurgical reconstruction of the right-sided hepatic vasculature. A strategy for preserving hepatic function in patients with proximal bile duct cancer. Langenbecks Arch Surg 2001;386:364-8. [Crossref] [PubMed]
- Yokoyama Y, Nagino M, Nishio H, et al. Recent advances in the treatment of hilar cholangiocarcinoma: portal vein embolization. J Hepatobiliary Pancreat Surg 2007;14:447-54. [Crossref] [PubMed]
- Koehler RE, Korobkin M, Lewis F. Arteriographic demonstration of collateral arterial supply to the liver after hepatic artery ligation. Radiology 1975;117:49-54. [Crossref] [PubMed]
- Liang G, Wen T, Mi K, et al. Resection of hilar cholangiocarcinoma combined with left hepatectomy and common hepatic arteriectomy without reconstruction. Hepatogastroenterology 2012;59:364-5. [PubMed]
- Yasuda Y, Larsen PN, Ishibashi T, et al. Resection of hilar cholangiocarcinoma with left hepatectomy after pre-operative embolization of the proper hepatic artery. HPB (Oxford) 2010;12:147-52. [Crossref] [PubMed]
- Bhangui P, Salloum C, Lim C, et al. Portal vein arterialization: a salvage procedure for a totally de-arterialized liver. The Paul Brousse Hospital experience. HPB (Oxford) 2014;16:723-38. [Crossref] [PubMed]
- Nimura Y, Hayakawa N, Kamiya J, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg 1990;14:535-43; discussion 44. [Crossref] [PubMed]
- Cheng QB, Yi B, Wang JH, et al. Resection with total caudate lobectomy confers survival benefit in hilar cholangiocarcinoma of Bismuth type III and IV. Eur J Surg Oncol 2012;38:1197-203. [Crossref] [PubMed]
- Kow AW, Wook CD, Song SC, et al. Role of caudate lobectomy in type III A and III B hilar cholangiocarcinoma: a 15-year experience in a tertiary institution. World J Surg 2012;36:1112-21. [Crossref] [PubMed]
- Wahab MA, Sultan AM, Salah T, et al. Caudate lobe resection with major hepatectomy for central cholangiocarcinoma: is it of value? Hepatogastroenterology 2012;59:321-4. [PubMed]
- Manfredi R, Barbaro B, Masselli G, et al. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis 2004;24:155-64. [Crossref] [PubMed]
- Cho ES, Park MS, Yu JS, et al. Biliary ductal involvement of hilar cholangiocarcinoma: multidetector computed tomography versus magnetic resonance cholangiography. J Comput Assist Tomogr 2007;31:72-8. [Crossref] [PubMed]
- Kato T, Tsukamoto E, Kuge Y, et al. Clinical role of (18)F-FDG PET for initial staging of patients with extrahepatic bile duct cancer. Eur J Nucl Med Mol Imaging 2002;29:1047-54. [Crossref] [PubMed]
- Cho MS, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg 2012;16:1672-9. [Crossref] [PubMed]
- Seyama Y, Makuuchi M. Current surgical treatment for bile duct cancer. World J Gastroenterol 2007;13:1505-15. [Crossref] [PubMed]
- Ebata T, Yokoyama Y, Igami T, et al. Review of hepatopancreatoduodenectomy for biliary cancer: an extended radical approach of Japanese origin. J Hepatobiliary Pancreat Sci 2014;21:550-5. [Crossref] [PubMed]
- Hirono S, Tani M, Kawai M, et al. Indication of hepatopancreatoduodenectomy for biliary tract cancer. World J Surg 2006;30:567-73; discussion 574-5. [Crossref] [PubMed]
- Miwa S, Kobayashi A, Akahane Y, et al. Is major hepatectomy with pancreatoduodenectomy justified for advanced biliary malignancy? J Hepatobiliary Pancreat Surg 2007;14:136-41. [Crossref] [PubMed]
- Wakai T, Shirai Y, Tsuchiya Y, et al. Combined major hepatectomy and pancreaticoduodenectomy for locally advanced biliary carcinoma: long-term results. World J Surg 2008;32:1067-74. [Crossref] [PubMed]
- Kaneoka Y, Yamaguchi A, Isogai M, et al. Survival benefit of hepatopancreatoduodenectomy for cholangiocarcinoma in comparison to hepatectomy or pancreatoduodenectomy. World J Surg 2010;34:2662-70. [Crossref] [PubMed]
- Hemming AW, Magliocca JF, Fujita S, et al. Combined resection of the liver and pancreas for malignancy. J Am Coll Surg 2010;210:808-14, 814-6.
- Ebata T, Yokoyama Y, Igami T, et al. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg 2012;256:297-305. [Crossref] [PubMed]
- Lim CS, Jang JY, Lee SE, et al. Reappraisal of hepatopancreatoduodenectomy as a treatment modality for bile duct and gallbladder cancer. J Gastrointest Surg 2012;16:1012-8. [Crossref] [PubMed]
- Sakamoto Y, Nara S, Kishi Y, et al. Is extended hemihepatectomy plus pancreaticoduodenectomy justified for advanced bile duct cancer and gallbladder cancer? Surgery 2013;153:794-800. [Crossref] [PubMed]
- Nagino M. Cutting edge of an aggressive surgical approach for perihilar cholangiocarcinoma. Updates Surg 2013;65:81-3. [Crossref] [PubMed]
- Bird NTE, McKenna A, Dodd J, et al. Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg 1999;230:808-18; discussion 819. [Crossref] [PubMed]
- He P, Shi JS, Chen WK, et al. Multivariate statistical analysis of clinicopathologic factors influencing survival of patients with bile duct carcinoma. World J Gastroenterol 2002;8:943-6. [Crossref] [PubMed]
- Sakamoto Y, Kosuge T, Shimada K, et al. Prognostic factors of surgical resection in middle and distal bile duct cancer: an analysis of 55 patients concerning the significance of ductal and radial margins. Surgery 2005;137:396-402. [Crossref] [PubMed]
- Hong SM, Pawlik TM, Cho H, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery 2009;146:250-7. [Crossref] [PubMed]
- Igami T, Nagino M, Oda K, et al. Clinicopathologic study of cholangiocarcinoma with superficial spread. Ann Surg 2009;249:296-302. [Crossref] [PubMed]
- Kawai M, Tani M, Kobayashi Y, et al. The ratio between metastatic and examined lymph nodes is an independent prognostic factor for patients with resectable middle and distal bile duct carcinoma. Am J Surg 2010;199:447-52. [Crossref] [PubMed]
- Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci 2010;17:476-89. [Crossref] [PubMed]
- Young AL, Prasad KR, Toogood GJ, et al. Surgical treatment of hilar cholangiocarcinoma in a new era: comparison among leading Eastern and Western centers, Leeds. J Hepatobiliary Pancreat Sci 2010;17:497-504. [Crossref] [PubMed]
- Li H, Qin Y, Cui Y, et al. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: a Chinese experience. Dig Surg 2011;28:226-31. [Crossref] [PubMed]
- Aoba T, Ebata T, Yokoyama Y, et al. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg 2013;257:718-25. [Crossref] [PubMed]
- Dumitrascu T, Chirita D, Ionescu M, et al. Resection for hilar cholangiocarcinoma: analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J Gastrointest Surg 2013;17:913-24. [Crossref] [PubMed]
- Furusawa N, Kobayashi A, Yokoyama T, et al. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg 2014;38:1164-76. [Crossref] [PubMed]
- Oguro S, Esaki M, Kishi Y, et al. Optimal indications for additional resection of the invasive cancer-positive proximal bile duct margin in cases of advanced perihilar cholangiocarcinoma. Ann Surg Oncol 2015;22:1915-24. [Crossref] [PubMed]
- Buettner S, van Vugt JL, Gani F, et al. A Comparison of Prognostic Schemes for Perihilar Cholangiocarcinoma. J Gastrointest Surg 2016;20:1716-24. [Crossref] [PubMed]
- Nakanishi Y, Tsuchikawa T, Okamura K, et al. Prognostic impact of the site of portal vein invasion in patients with surgically resected perihilar cholangiocarcinoma. Surgery 2016;159:1511-9. [Crossref] [PubMed]
- Groot Koerkamp B, Wiggers JK, Allen PJ, et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J Am Coll Surg 2015;221:1041-9. [Crossref] [PubMed]
- Komaya K, Ebata T, Yokoyama Y, et al. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery 2018;163:732-8. [Crossref] [PubMed]
- Zhang XF, Beal EW, Chakedis J, et al. Defining Early Recurrence of Hilar Cholangiocarcinoma After Curative-intent Surgery: A Multi-institutional Study from the US Extrahepatic Biliary Malignancy Consortium. World J Surg 2018;42:2919-29. [Crossref] [PubMed]
- Tabata M, Kawarada Y, Yokoi H, et al. Surgical treatment for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2000;7:148-54. [Crossref] [PubMed]
- Juntermanns B, Fingas CD, Sotiropoulos GC, et al. Klatskin tumor: long-term survival following surgery. Chirurg 2016;87:514-9. [Crossref] [PubMed]
- Groot Koerkamp B, Wiggers JK, Allen PJ, et al. American Joint Committee on Cancer staging for resected perihilar cholangiocarcinoma: a comparison of the 6th and 7th editions. HPB (Oxford) 2014;16:1074-82.
- Chaiteerakij R, Harmsen WS, Marrero CR, et al. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol 2014;109:1881-90. [Crossref] [PubMed]
- Edeline J, Bonnetain F, Phelip JM, et al. Gemox versus surveillance following surgery of localized biliary tract cancer: Results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol 2017;35:225. [Crossref]
- Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: The BILCAP randomized study. J Clin Oncol 2017;35:4006. [Crossref]
- Stein A, Arnold D, Bridgewater J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015;15:564. [Crossref] [PubMed]
- Jung JH, Lee HJ, Lee HS, et al. Benefit of neoadjuvant concurrent chemoradiotherapy for locally advanced perihilar cholangiocarcinoma. World J Gastroenterol 2017;23:3301-8. [Crossref] [PubMed]
Cite this article as: Capobianco I, Rolinger J, Nadalin S. Resection for Klatskin tumors: technical complexities and results. Transl Gastroenterol Hepatol 2018;3:69.


