The current role of laparoscopic resection for HCC: a systematic review of past ten years
Introduction
Hepatocellular carcinoma (HCC) is the sixth most frequent primary cancer and the third cause of cancer-related deaths worldwide, with 782 000 diagnoses in 2012 and a rising incidence (1). According to Barcelona Clinic Liver Cancer (BCLC) staging system, liver resection represents the first choice for treatment of patients with very early and early HCC (BCLC stage 0–A) (2). About 80–90% of HCCs arise in a cirrhotic liver (1), therefore any surgical procedure in these patients implies higher risks of complications, both intra-operative (e.g., bleeding) and post-operative (e.g., post hepatectomy liver failure, PHLF) (3). Before 1991, when first laparoscopic liver resection (LLR) was described (4), open approach was the only choice for liver surgery. Since then, LLR spread exponentially: initially only non-anatomical wedge resections were considered as a safe procedure; later on, anatomical resections were introduced and today specialized centres perform also major hepatectomies. At present, more than 9,000 LLRs are described in literature (5), but most of them are minor resections of subcapsular lesions or lesions located in the left lobe. For these lesions, laparoscopy is now considered a standard practice. Major hepatectomies and resections of posterosuperior segments, instead, are still regarded as innovative procedures that need to be fully developed (6). Since open liver resection (OLR) represents the standard technique, it is crucial to determine advantages and disadvantages of LLR versus OLR. Unfortunately, no randomized controlled trial has been published up to now, therefore the quality of evidence in this field is low, mainly based on case series and case-matched analyses. In order to define the current role of LLR and to establish guidelines, the Second International Consensus Conference on LLR was held in 2014, in Morioka (7). According to Morioka’s statements, LLR does not show inferior results considering mortality, overall survival (OS), negativity of margins, and costs, while seems to be superior in term of length of hospitalization and number of intra-operative blood transfusions. Morbidity rates in LLR are inferior when major resections only are considered. Minimally invasive surgery reduces the rate of decompensation in cirrhotic patients and adherence formation, the latter being a desirable goal in view of an eventual salvage liver transplantation (6). Further advantages are given by image magnification allowed by the endoscope and better aesthetical results. On the other side, LLR requires higher skills (e.g., suturing skills) and shows some technical limitations such as lack of tactile feedback, restriction of movements, and presence of physiologic tremors. Furthermore, resections of lesions located in posterosuperior segments (I, IVa, VII and VIII) still constitute a big challenge even for expert surgeons. Finally, difficult haemorrhage control is the main reason of conversions to laparotomic approach (6). However, some of these limitations might be overcome by the introduction of robotic surgery. In fact, this new minimally invasive technique guarantees stability, increases freedom of movement thanks to instrument flexibility and introduces a three-dimensional vision with depth perception (8).
The aim of this review is to systematically assess short-term (morbidity and mortality) and long-term (OS and disease-free survivals) major outcomes in patients who underwent LLR for HCC.
Materials and methods
We conducted an advanced PubMed research to select articles of interest. The following keywords were used to search in titles or abstracts: “HCC” or “hepatocellular carcinoma” and “liver resection” or “surgery” or “hepatectomy” and “laparoscopic” or “minimally invasive” or “robotic”. Our enquiry was restricted to English articles, published from January 2007 to December 2017 and for which full text was available. We applied the “article types” filter to select “clinical trial”, “comparative study”, “multicenter study” and “validation study”. A total of 86 remaining articles were considered for revision. We included all retrospective, prospective, and comparative clinical trials reporting short-term major outcomes and long-term outcomes of any series of patients with diagnosis of HCC who underwent laparoscopic or robotic resection. Morbidity and mortality were considered for evaluation of short-term outcomes, while long-term outcomes were expressed in terms of overall survival (OS) and disease-free survival (DFS). Data related to intra-operative outcomes (blood loss, total operation time and duration of surgery) and post-operative outcomes (duration of hospitalization) were collected, although not being the main object of this review. Among comparative studies, we included only those comparing different surgical techniques (e.g., laparoscopic versus robotic), while we excluded those comparing laparoscopies to all other curative options for HCC (e.g., ablative treatments). Studies were excluded if they: (I) were reviews, meta-analyses or case reports; (II) included patients resected after receiving locoregional treatments for the same tumor; (III) included patients resected for lesions other than HCC (e.g., metastatic tumors); (IV) included laparoscopic-assisted procedures; (V) considered series of highly selected patients (e.g., obese patients or elderly patients); (VI) did not report any of the outcome considered in this review; (VII) full text was not available.
When more than one article was reported by the same institution and/or authors, we selected either the one with the largest series or the most recent, with the exception of multicenter studies. We considered patients after propensity-score matching (PSM) whenever it was performed.
Laparoscopic resections were included when performed with pure laparoscopic or hand-assisted techniques. Pure laparoscopic resection’s essential features are pneumoperitoneum induction, generally through a periumbilical incision, and ports placing (usually three or four ports from 5 to 12 mm), followed by intra-abdominal parenchymal transection; at the end of the resection a larger incision is performed in order to retrieve the specimen from abdominal cavity, most frequently through an enlarged port site or a new incision, such as Pfannenstiel incision. For hand-assisted technique, besides ports for laparoscopic instruments, a further incision is performed through the abdominal wall for the placement of a hand port, allowing the introduction of the operator’s hand in the abdomen for manual palpation of the various structures and for parenchyma retraction during transection. Laparoscopic-assisted liver resections were not considered as “laparoscopic” procedures in this review.
Pre-operative diagnosis of HCC was based on invasive (i.e., biopsy) or non-invasive European Association for the Study of the Liver (EASL) criteria (9). The percentage of patients with liver cirrhosis at pathology was reported for each study whenever available. Mortality is considered at 30- or 90-day postsurgery or intra-hospital. We reported the overall complication rate for each study and, whenever available, we also reported complication rates classified according to type (surgical vs. non-surgical) and severity [grade I–II or ≥ III according to Clavien-Dindo classification (10)]. Wound infections, biliary fistula, liver failure, portal thrombosis, abscesses and other conditions were considered as surgical complications. General postoperative complications such as pulmonary complications, renal failure, and ileus or cardiac arrhythmia were considered as non-surgical complications.
Results
Study and patients characteristics
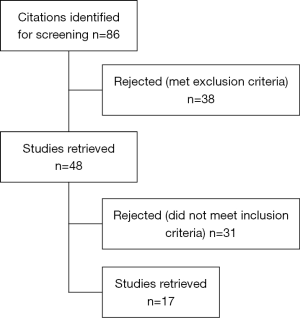
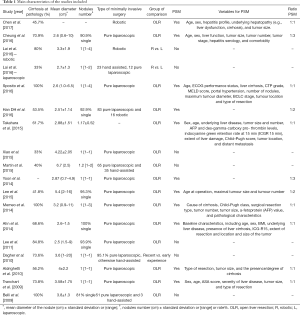
A total of 1,501 patients from 17 studies were included. The selection process is shown in Figure 1. Table 1 illustrates the main characteristics of the studies. Among the studies selected, all are retrospective and 15 out of 17 compare LLR with OLR; one compares robotic and laparoscopic techniques and one compares recent and early experience of LLR. PSM analysis was used in 11 studies to compare cohorts of patients who underwent LLR vs. OLR; variables used for the PSM and PSM ratio are reported in Table 1. A total of 975 patients were included after PSM.
Full table
The majority of patients underwent pure laparoscopic resection, while robotic and hand-assisted techniques were less represented. Presence of cirrhosis at pathology ranged from 33% to 100%; in 3 studies cirrhosis was an inclusion criteria (142 patients). All the patients included had a good liver function, resulting in Child-Pugh class A or normal liver function without portal hypertension for most of them. Among the studies included, 6 were conducted on patients with single nodule of HCC and in the other series the majority of patients had only one nodule of HCC. The mean diameter of the major nodule or of the single nodule ranged from 2.5 to 6.7 cm (Table 1).
The extension of resections ranged from subsegmentectomies to major hepatectomies. The tumours were located in any part of the liver; thus, resections involved every segment including the posterosuperior ones.
Short-term outcomes
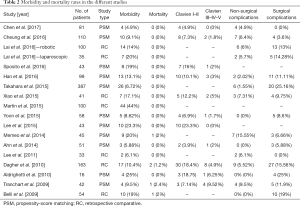
Table 2 shows short-term major outcomes for each study. Mortality rates ranged from 0% to 2.4% with 13 series out of 17 reporting 0% mortality. Considering all the studies, 5 patients died in the perioperative period: two of them for acute respiratory distress syndrome, one for liver failure and one for tumour progression. For one patient the cause of death was not reported.
Full table
Overall morbidity rates ranged from a minimum of 4.9% to a maximum of 44%. Classification according to Clavien-Dindo was available for 11 studies and in these series most of the post-operative complications fell in Clavien grade I and II (range: 3.9–23.3%), while grades III to V were a minority (range: 0–9.52%). A total of 15 studies reported also surgical vs. non-surgical complications; the maximum rate of non-surgical complications was 15.55%, while the maximum rate of surgical complication was 25.16%.
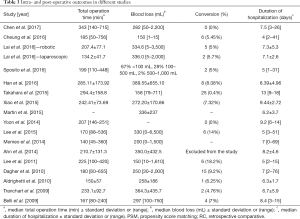
Considering intra-operative and post-operative outcomes of morbidity, the median blood loss ranged from 150 to 389 mL, while the maximum rate of conversion reported was 18.2%, with one study excluding patients for whom conversion was needed and two studies not reporting conversion rates. The median duration of surgery ranged from 134 to 343 minutes and the median duration of hospitalization ranged from 4 to 13 days (Table 3).
Full table
Long-term outcomes
Table 4 shows long-term outcomes in the selected publications. The ranges of overall survival rates at 1-, 3- and 5-year were 72.8–100%, 60.7–93.5% and 38–89.7% respectively. The ranges of disease free survival rates at 1-, 3- and 5-year were 45.5–91.5%, 20–72.2% and 19–67.8% respectively. One study reported survivals in term of mean months, with 40.2 and 23.3 months for OS and DFS respectively.
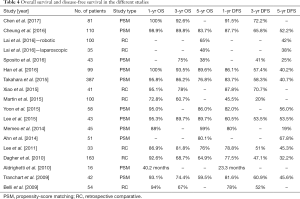
Full table
Discussion
The progressive spread of minimally invasive liver surgery and the development of new dedicated technologies have led to the need for redefining the role of laparoscopy in the field of liver resection. In this review we focused on short term and long-term major outcomes of LLR for HCC in order to illustrate the feasibility and the advantages of minimally invasive liver surgery.
Although randomized controlled trials (RCT) would be the gold standard to obtain the highest quality of evidence, ethical and practical aspects make it difficult to set them up. Therefore, no randomized prospective clinical trial has been published yet and all the available data derive mainly from retrospective case-control studies, reviews or meta-analysis.
In view of the selected publications, that include large series of matched patients [e.g., Takahara et al. (11), 387 patients], laparoscopy shows comparable results to OLR for what concerns long-term oncologic outcomes (OS and DFS) and mortality, but superior results in term of morbidity and intra-operative outcomes.
The mortality rates are very low, with 13 series having 0% mortality and only 5 unfavourable events among 1,501 patients. Overall morbidity does not exceed 25%, with the exception of the study by Martin et al. (12) in which the morbidity rate amounts to 44% in LLR, anyway it was not found to be significantly different from the morbidity rate in OLR. This difference may be attributed to the time interval during which LLRs were performed; in fact, even if the study was published in 2015, patients included were resected from 2004 to 2009. For what concerns complications’ severity, only Tranchart et al. (13) reported a rate of complication classified as Clavien grade ≥ III higher than those classified as Clavien I–II; in all other study, the rate of complications classified as Clavien I–II is higher. Considering intra-operative outcomes, no substantial difference was found in blood loss; while for what concerns median operation time the longest duration, reported by Chen et al. (14) (343 min), might be mostly attributable to the need for extra time for the robot docking. In two studies the conversion rates exceeded 10% [Lee et al. (15) and Lee et al. (16), respectively 14% and 18.2%] and were due to uncontrolled bleeding. Finally, the study by Takahara et al. (11) reported the longest duration of hospitalization with a median of 13 days, but it resulted significantly shorter (P<0.001) than for the OLR patients anyway.
Overall, our results are in line with recent meta-analysis (6,17-19) which have demonstrated that LLR has shorter operation time, lower complications’ rates, reduced blood loss, reduced need for transfusions, and, consequently, a shorter hospital stay. Furthermore, other benefits can derive from minimally invasive surgery. Of note, all three comparative studies conducted solely on patients with proven cirrhosis found that morbidity was significantly lower in the LLR group than in OLR group. In fact, patients with liver cirrhosis represent a fragile category and they could take huge advantage from LLR, since it is associated to a lower incidence of postoperative ascites and liver failure (20-23). Indeed, cirrhotic patients are exposed to a higher risk of decompensation also attributable to surgery-induced injury. The risk of decompensation can be appreciably minimized when choosing a minimally invasive approach, in which round ligament and venous collateral circulation are preserved and a smaller abdominal incision is performed. Based on these considerations, LLR should be the first choice in patients with liver cirrhosis with resectable HCC.
LLR also allows the reduction of the adhesions due to previous liver resection, that may be responsible for a remarkable increase of the surgical difficulty of a salvage liver transplantation (LT) in case of recurrence. A study by Laurent et al. (24) comparing intra-operative LT after ORL and LLR, showed that the latter was associated with a reduced blood loss, a reduced need for transfusions and a shorter duration of the hepatectomy phase and whole LT. Consequently, LLR should be preferred in patients listed for LT or in patients for whom LT is a possible option in case of recurrence (25,26).
Robotic liver resection (RLR) is emerging as a valid alternative to laparoscopic and OLR, a strong point being its ability to overcome some evident limits of the other techniques. Still, robotic surgery encounters operators’ resistance mainly due to the lack of evidence of comparable oncologic outcomes. Our review includes two of the largest series of RLR for HCC, none of which found any difference in OS and DFS between RLR and ORL and between RLR and LLR. Even if literature in this field is scarce, RLR seems to be at least not inferior to other approaches, but brings the advantages of robotic surgery such as a three-dimensional vision, tremor filtration and the possibility of intra-corporeal suturing. This consideration seem to be applicable also to patients with liver cirrhosis, as Di Sandro et al. (27) found that totally RLR for single HCC on cirrhosis had shorter operative time if console time only is considered, reduced blood loss and less need of packed RBC and FFP transfusions.
The study conducted by Dagher et al. (28) included an analysis of results separating the first 25 resections from each centre (early experience) versus the latter resections (recent experience), showing a significant improvement of surgical and postoperative results in the recent experience group. These study underlines the importance of minimally invasive surgery being performed in experienced centres, specially for lesions located in posterosuperior segments and for major resections (29,30). In this regard, a recent analysis demonstrated robotic surgery to have a shorter learning curve if compared to laparoscopy (31), with only 16 procedures required to significantly increase difficulty index of robotic procedures; thus, the implementation of robotic surgery can be useful in order to rapidly and safely expand indications for complex liver resections.
In conclusion, our review confirms the superiority of LLR compared to ORL regarding perioperative outcomes without compromising long-term outcomes, especially for cirrhotic patients. Robotic surgery overcomes some weaknesses of laparoscopy and its use should be encouraged in order to perform difficult procedures.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Llovet JM, Fuster J, Bruix J. Prognosis of Hepatocellular Carcinoma. Hepatogastroenterology 2002;49:7-11. [PubMed]
- Fukushima K, Fukumoto T, Kuramitsu K, et al. Assessment of ISGLS Definition of Posthepatectomy Liver Failure and Its Effect on Outcome in Patients with Hepatocellular Carcinoma. J Gastrointest Surg 2014;18:729-36. [Crossref] [PubMed]
- Reich H, McGlynn F, DeCaprio J, et al. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956-8. [PubMed]
- Ciria R, Cherqui D, Geller DA, et al. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Yin Z, Fan X, Ye H, et al. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: A global systematic review and meta-analysis. Ann Surg Oncol 2013;20:1203-15. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in morioka. Ann Surg 2015;261:619-29. [PubMed]
- Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic Surgery: A Current Perspective. Ann Surg 2004;239:14-21. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 2015;22:721-7. [Crossref] [PubMed]
- Martin RCG, Mbah NA, Hill RS, et al. Laparoscopic versus open hepatic resection for hepatocellular carcinoma: Improvement in outcomes and similar cost. World J Surg 2015;39:1519-26. [Crossref] [PubMed]
- Tranchart H, Di Giuro G, Lainas P, et al. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc 2010;24:1170-6. [Crossref] [PubMed]
- Chen PD, Wu CY, Hu RH, et al. Robotic Versus Open Hepatectomy for Hepatocellular Carcinoma: A Matched Comparison. Ann Surg Oncol 2017;24:1021-8. [Crossref] [PubMed]
- Lee JJ, Conneely JB, Smoot RL, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma at a North-American Centre: A 2-to-1 matched pair analysis. HPB 2015;17:304-10. [Crossref] [PubMed]
- Lee KF, Chong CN, Wong J, et al. Long-term results: Of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: A case-matched analysis. World J Surg 2011;35:2268-74. [Crossref] [PubMed]
- Morise Z, Ciria R, Cherqui D, et al. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci 2015;22:342-52. [Crossref] [PubMed]
- Parks KR, Kuo YH, Davis JM, et al. Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB (Oxford) 2014;16:109-18. [Crossref] [PubMed]
- Zhou YM, Shao WY, Zhao YF, et al. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci 2011;56:1937-43. [Crossref] [PubMed]
- Mirnezami R, Mirnezami AH, Chandrakumaran K, et al. Short- and long-term outcomes after laparoscopic and open hepatic resection: Systematic review and meta-analysis. HPB 2011;13:295-308. [Crossref] [PubMed]
- Sposito C, Battiston C, Facciorusso A, et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 2016;103:871-80. [Crossref] [PubMed]
- Memeo R, De’Angelis N, Compagnon P, et al. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: A case-control study. World J Surg 2014;38:2919-26. [Crossref] [PubMed]
- Belli G, Limongelli P, Fantini C, et al. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg 2009;96:1041-8. [Crossref] [PubMed]
- Laurent A, Tayar C, Andréoletti M, et al. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2009;16:310-4. [Crossref] [PubMed]
- De Carlis L, Di Sandro S, Giacomoni A, et al. Liver transplantation for hepatocellular carcinoma recurrence after liver resection: why deny this chance of cure? J Clin Gastroenterol 2013;47:352-8. [Crossref] [PubMed]
- Hu Z, Wang W, Li Z, et al. Recipient outcomes of salvage liver transplantation versus primary liver transplantation: A systematic review and meta-analysis. Liver Transpl 2012;18:1316-23. [Crossref] [PubMed]
- Di Sandro S, Lauterio A, Giacomoni A, et al. Totally robotic liver resection for hepatocellular carcinoma in cirrhotic patients: safety and feasibility. J Robot Surg 2014;8:357-64. [Crossref]
- Dagher I, Belli G, Fantini C, et al. Laparoscopic Hepatectomy for Hepatocellular Carcinoma: A European Experience. J Am Coll Surg 2010;211:16-23. [Crossref] [PubMed]
- Ishizawa T, Gumbs AA, Kokudo N, et al. Laparoscopic segmentectomy of the liver: From segment I to VIII. Ann Surg 2012;256:959-64. [Crossref] [PubMed]
- Dagher I, Gayet B, Tzanis D, et al. International experience for laparoscopic major liver resection. J Hepatobiliary Pancreat Sci 2014;21:732-6. [Crossref] [PubMed]
- Efanov M, Alikhanov R, Tsvirkun V, et al. Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB 2017;19:818-24. [Crossref] [PubMed]
Cite this article as: Di Sandro S, Danieli M, Ferla F, Lauterio A, De Carlis R, Benuzzi L, Buscemi V, Pezzoli I, De Carlis L. The current role of laparoscopic resection for HCC: a systematic review of past ten years. Transl Gastroenterol Hepatol 2018;3:68.