Hepatocellular carcinoma in children: hepatic resection and liver transplantation
Introduction
Primary liver tumors are uncommon in childhood, representing approximately 1% of pediatric solid malignancies. Hepatocellular carcinoma (HCC) is the second most common pediatric primary hepatic tumor accounting 27% of all liver malignancy and following hepatoblastoma (HB) (70% of all pediatric liver tumors) (1). In children, HCC has an incidence of 0.7/1,000,000 per year, being much rarer than in adults (7.5–20/1,000,000 per year) (2,3). Recent data from the Surveillance, Epidemiology, and End Results (SEER) database shows that 99.6% of HCC occurs in adults, while only 0.4% in pediatric patients; moreover, the incidence of HCC is significantly higher in countries with endemic hepatitis B infection, such as in Eastern and Southeastern Asia and in Africa, for both pediatrics and adult population (4).
Despite HCC in adults appears mainly on the background of cirrhotic liver, HCC in children may occur in two different biological patterns. The majority of pediatric HCC (70%) arise on a normal liver and may present histological features such as: (I) conventional HCC; (II) transitional type of tumor with features of both HCC and HB defined as “Hepatocellular Neoplasm not otherwise specifies” (HCN-NOS) (5); (III) fibrolamellar HCC. The remainder 30% of pediatric HCC occurs in a contest of chronic liver disease such as cirrhosis, metabolic disorders, chronic cholestasis and chronic viral hepatitis (2,3). Pediatric HCC is typically diagnosed in older children and adolescents, accounting more than 80% of primary hepatic tumors between 15 and 19 years of age (3). Clinical signs and symptoms of pediatric HCC include abdominal mass and pain, hepatosplenomegaly, gastric reflux and, in advanced cases, cachexia and jaundice; additionally, in a context of chronic liver disease signs of liver insufficiency may occur (3). Only 55–65% of children with HCC have elevated blood level of alpha-fetoprotein (AFP), while in a quarter of patients the AFP might be normal (6). At presentation children usually have more advanced tumoral disease compared to adult patients, characterized by more frequent distant disease (33.1% vs. 20.8%, P<0.001) and lower rate of localized tumors (28.1% vs. 48%, P<0.001) (4). Pediatric HCC has also larger tumor size at the time of detection, being >4 cm in 79.6% of cases in children while 62% in adults (P=0.02) (4).
Since the peculiar pattern of HCC in children, historically different staging systems have been proposed to classify pediatric HCC including the TNM system, commonly used in adults, and the Pretreatment Extension of Disease (PRETEXT) system. The PRETEXT system, which is a tumor extension and risk stratification system for HB introduced in 1990 by the International Society of Pediatric Oncology Liver group (SIOPEL) is based on Couinaud’s segmentation of liver and divides the organ into 4 sectors; based on the number of sectors involved by the tumor, PRETEXT is defined PRETEXT I to IV, and further annotations include involvement of portal system, hepatic veins, extra hepatic lesions and metastasis (7).
The management of pediatric HCC remains difficult since its clinical presentation and histological patterns differ both from adult HCC as well as from pediatric HB. First of all, pediatric HCC occurs mainly in normal liver and falls out-side of the “classical” criteria for surgical resection used in adults HCC (8). Moreover, the well-known risk factors (tumor size, vascular invasion, multiple tumors and fibrolamellar variant) identified for tumor recurrence in adult HCC showed different significance in terms of prognosis in children (9). Secondly, despite HCC has been considered a non-chemosensitive tumor in adults, generally a better response to chemotherapy is observed in pediatric HCC (10), leading to the assumption that HCC in children may be a subgroup of transitional tumors consisting of both HB and HCC tumor variants (11,12). Therefore, the treatment of pediatric HCC has evolved by the large experience of HCC in adults combined with lessons learned by the management of HB in children.
In adults, since HCC usually occurs in cirrhotic liver, the surgical treatment is defined based on tumor characteristics (size, number of lesions, vascular invasion) and a composite assessment of liver function, portal hypertension, extent of hepatectomy, expected volume of the future liver remaining, performance status and patient’s co-morbidities. In adult HCC, liver resection is indicated for single HCC of any size (particularly <2 cm) when hepatic function is preserved and sufficient remaining liver volume is maintained (13); liver transplantation (LT) is recommended as first-line option for HCC unsuitable for resection within Milan criteria (single tumor smaller than 5 cm, or in case of many tumors, no more than 3, and each not exceeding 3 cm in diameter), achieving 5-year disease-free survivals of 80% after LT (13,14). International guidelines suggest that the Milan criteria are considered the benchmarks for selection of patients with HCC candidate to LT and the basis for comparison with other less restrictive criteria, which achieved satisfactory results in selected HCC population (15). Despite the role of loco-regional treatments, such as transarterial chemoembolization (TACE), before LT has not been fully established in adults yet, these are commonly used as “bridge” strategy in Milan-in criteria HCC patients with an expected LT-waiting time >6 months, or as “downstage” therapy for patient beyond Milan criteria, who might be considered eligible for LT after successful “downstage” treatment (16,17).
In children, several studies showed that the only curative treatment of HCC consists in the radical resection of the tumor, weather by partial liver resection or LT (8,18-23). As in adults, the surgical resection represents the cornerstone of HCC treatment, thus pediatric HCC management requires a more complex multi-disciplinary strategy (3). The overall 5-year survival rate for children with HCC is reported approximately 20–30% in different trials, but according to the HCC treatment, the survival rate is 50–60% for children undergoing upfront surgical resection (reaching up to 70–80% for these treated with LT), 20–30% for patients receiving combination of surgery and chemotherapy (often with more advanced disease at diagnosis), while 0–12% for children who do not receive any therapy (8,18,19,20). Recent SEER data showed that surgery represents the principal treatment for pediatric HCC (48.6%), followed by radiation alone (3.2%) and combination of surgery and radiation (0.8%), thus 47.4% of patients don’t receive any treatment (4).
While complete tumor resection remains the only chance for cure and prolonged survival, at time of diagnosis nearly 80% of pediatric HCC are unresectable due to large and multiple lesions and/or presence of metastasis (3,4,18,21). To face this problem, in the last years great interest has been posed in making unresectable HCC in resectable tumors by extending criteria for surgical treatment and/or associating multi-modal treatments, such as systemic chemotherapy and local-regional therapy, however no universal criteria for the management of pediatric HCC has been defined yet.
Pediatric HCC in normal liver
The majority (70%) of pediatric HCC occurs in normal liver. Theoretically, the absence of concomitant chronic liver disease should allow tolerating significant anatomic resection due to adequate hepatic reserve, while reserving LT for unresectable tumors, especially in face of organ’s shortage (22). However, at the time of diagnosis less than 20% of pediatric HCC are considered eligible for primary resection due to advanced stage (i.e., multifocal involvement, vascular encasement, extra-hepatic lesions) (23).
Several studies suggest that when possible the upfront surgical treatment, including liver resection or LT, is the preferable choice in pediatric HCC without concomitant liver disease, representing the treatment of choice in 48–52% of patients (3,4,18). In 2014, a retrospective SEER study analyzing 218 children with primary HCC (concomitant liver disease not specified) showed that complete tumor resection significantly improved the overall survival at 5 years compared to no surgery (60% vs. 0% respectively, P<0.0001) and that fibrolamellar HCC variant had a greater survival compared to the non-fibrolamellar HCC after 10 years of follow-up (59% vs. 37%, P=0.002) (18). The SEER data revealed also that after resection, children have better survival than adults (13.1 vs. 8.3 years, P<0.001), primarily attributable to the more aggressive surgical intervention and the favorable fibrolamellar histologic variant in the pediatric population (4). Children undergoing primary resection expected a 5-year survival rate of up to 50% and a recurrence rate of 20–30%, confirming that tumor resection is the most important factor for survival, while lymphovascular invasion, extra-hepatic or metastatic disease are poor prognostic factors (<10% 5-year disease-free survival) (4).
Recently, also Wang et al. underlined that, when technically feasible, surgical resection is the recommended primary treatment for HCC, being associated with superior outcomes compared to alternative treatment such as TACE or other supportive management (24). Interestingly, patients who underwent TACE for advanced-stage HCC had better overall survival compared to these not receiving treatment, evoking a beneficial role of loco-regional treatment for advanced pediatric HCC as already described in adults (25).
Since the radical resection of HCC showed the best results, different protocols of neoadjuvant chemotherapy have been proposed to convert unresectable tumors (due to advanced loco-regional disease and/or metastasis) into resectable tumors (26-29).
Evidence of chemo-response associated to surgical resection led in the last decades to develop comprehensive protocol such as the SIOPEL, which combine chemotherapy and surgical treatment (26). The SIOPEL 1 trial showed that nearly 50% of advanced pediatric HCC had a partial response to chemotherapy based on cisplatin and doxorubicin (PLADO), but survival improved by less than 5% among responders (19,26). Recently, the SIOPEL 2 and 3 trials, based on the intensification of platinum agents regimen, reported outcomes of 85 HCC children: 13 (15%) patients received primary surgery [of whom 12 (92%) had a complete resection (R0)]; 72 (85%) children were treated with neoadjuvant chemotherapy, of whom 33 (45.8%) patients achieved delayed surgery [15 (45%) obtained complete tumor resectability (R0), while 17 (52%) incomplete resection (R1) or microscopic residual disease], but 29 (40.3%) children were not resectable after neoadjuvant chemotherapy (19). These results led Murawski et al. to conclude that the intensification of platinum agents in SIOPEL 2 and 3 trials doesn’t improve survivals and new treatment strategies for pediatric HCC, based on a better tumor shrinkage, need to be explored in order to facilitate surgery and prevent tumor recurrence (19). So far, there is a lack of trials comparing outcomes of patients with resectable HCC undergoing upfront surgery and children receiving neoadjuvant chemotherapy and then delayed surgery.
Whether sorafenib has a survival benefit in the pediatric population remains unclear since its application has been limited up to now (30). In adults with advanced HCC, the use of sorafenib demonstrated antitumoral activity, reducing tumor progression (31,32). However, in a randomized adult multicentre trial, sorafenib didn’t show any effectiveness as an adjuvant treatment following tumor resection or ablation (33). Recently, the first pediatric series treated with sorafenib was reported: 6/12 (50%) children with advanced HCC received sorafenib/PLADO followed by liver resection (n=4) or LT (n=2) and are recurrence-free after 20 months (34). Interestingly, out of 7 patients with unresectable tumor at diagnosis 3 (43%) became resectable and all patients with elevated AFP levels had a marked drop after 2 cycles. Based on these initial promising experiences, further data are needed to evaluate pharmacokinetics and to determine the appropriate dose of sorafenib in children.
These data suggest that the use of neoadjuvant therapy is on one hand useful to evaluate the HCC tumoral biological response (based on evolution of tumor size, metastasis and AFP levels), but on the other it doesn’t gain much on tumor resectability in almost half of treated children, in whom surgical intervention shouldn’t be delayed, since represents the only curative treatment for HCC.
In the last decades LT has been proposed as the most radical and curative therapy in unresectable pediatric HCC without extra-hepatic malignancy, which represents up to 80% of children with HCC at the time of presentation (35,36). In adults, the role of LT for HCC has been studied extensively with the adoption of the Milan criteria as well as other expanded criteria (such as the University of California, San Francisco) to determine transplant candidacy (13-15). On the contrary, in pediatric HCC without concomitant liver disease there are not universal selection criteria for LT: the American Association for Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) recommend that the indication for LT in childhood HCC must be discussed individually for each patient (37).
After the initial data from the United Network for Organ Sharing (UNOS) experience (38) showing inferior LT outcomes for pediatric HCC in comparison with all other pediatric liver tumors (5-year survival rate: HCC 53.7%, HB 72.7%, hemangioendothelioma 60.6%, non-tumors 84.4%.), more recent studies reported that LT in HCC children may achieve excellent results, with a 5-year survival rate up to 70–80% (Table 1). In 2010, Kosola et al. (39) analyzed the long-term outcomes of 6 children with HCC out of Milan criteria undergoing LT after neoadjuvant chemotherapy, reporting a 5-year and 10-year survival rate of 80% and 67%, respectively; in this series, the PRETEXT tumor staging had no effect on survival, while incomplete AFP response to chemotherapy and rescue LT were associated with decreased survival. Pham and colleagues (9) also reported excellent long term results after LT and chemotherapy for pediatric HCC, with a disease-free survival rate of 78% after 10 years of follow-up; the only risk factor associated with tumor recurrence was older age (mean age 18 vs. 11 years, P=0.04) at the time of transplantation, while Milan criteria in/out, tumor size, vascular invasion, multiple nodules, or fibrolamellar variant didn’t impact on HCC recurrence, in contrast to the well-known risks these factors hold in adults (46). As common practice in adults, in this series TACE was successfully performed when the time on LT waiting list was prolonged as “bridge” treatment (9). In children, the use of TACE (in combination or not with systemic chemotherapy) was recently reported also to successfully “downstage” tumors initially not candidable to LT (42,43). These single-center pediatric cases and the largest experience in adults led to consider that loco-regional treatment (such as TACE or radioablation) might be routinely considered also in pediatric HCC not only as “bridge” treatment for children awaiting LT, but also as “downstage” therapy for advanced HCC patients not candidable to transplantation at diagnosis (22,47).
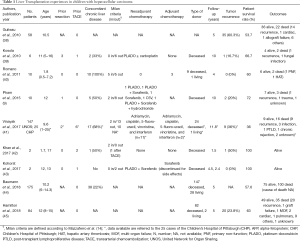
Full table
Recently, Hamilton and colleagues reported an update of the UNOS experience, observing a significant increase of survival rates in the last years after LT: HCC children transplanted from 2009 to 2012 had a 3-year graft and patient survival of 84% respectively, which were comparable to graft and patient survival of HB transplant patients (85% and 85% respectively, P=0.632), thus HCC patients had an increased risk of tumor recurrence (45). Notwithstanding this experience provided important information on the overall survivals of LT in HCC children, as the nature of transplant registry, no data are available regarding stage of tumoral disease at the time of transplantation, neoadjuvant/adjuvant chemotherapy, association with concomitant chronic liver disease, whether a patient underwent a primary LT or a rescue transplant following attempted resection; therefore, these results should be integrated with other international registries, such as the Pediatric Liver Unresectable Tumor Observatory (PLUTO) (48), for a fully interpretation.
Although some have suggested using the adult Milan criteria also in pediatric patients, the good results of LT for pediatric HCC outside of Milan criteria suggest that specific criteria to select children undergoing LT for HCC on a background of normal liver needs to be defined (8,49,50).
So far, few studies compared the post-operative outcomes of LT and liver resection in children with HCC (Table 2). Ismail et al. firstly showed that HCC children undergoing surgical resection have a significantly inferior survival rate compared to those treated by LT (40% vs. 72%, respectively), despite 73% of patients transplanted didn’t fulfill the Milan criteria (37). The favorable outcomes of LT over surgical resection in HCC children was also confirmed by the Pittsburgh group, suggesting that if the chemotherapy is unable to downstage the tumor, LT becomes the best option (21). In 2013, McAteer and colleagues (20) reported the outcomes of 80 children with HCC (60 liver resection vs. 20 LT) from the SEER registry, showing that the 5-year survival rate was 53.4% after resection and 85.3% after LT (HR =0.05, 95% CI, 0.003–0.094). In the last years, data have been showing excellent outcomes of LT for pediatric HCC while still limited conversion rate of unresectable tumors in resectable tumors by neoadjuvant therapy, consequently it is questionable if children with locally advanced HCC tumors, without extra-hepatic disease, should be considered for LT as first line of treatment.

Full table
In conclusion, the keystone of HCC treatment in children without chronic liver disease is the radical resection of the lesion. While non-metastatic locally advanced tumors are a current indication to LT, the satisfactory outcomes of LT for pediatric HCC may encourage to explore if the potentially resectable HCC might benefit from transplantation as first line of treatment, however further data are needed.
HCC in chronic liver disease
Despite in adults HCC is frequently diagnosed on the background of liver cirrhosis, in children only 30% of HCC is associated to underlying chronic liver disease including chronic viral hepatitis (hepatitis B and C), cholestatic liver disease (Alagille’s syndrome), metabolic diseases (glycogen storage disease, hereditary tyrosinemia, Wilson’s disease, hemochromatosis, alpha-1 antitrypsin deficiency) and autoimmune (autoimmune hepatitis, primary sclerosing cholangitis) liver disorders. Rarely, HCC has been reported in children affected by familial cholestatic syndromes, transaldolase deficiency, ataxia telangiectasia, Gardner’s syndrome or familial adenomatous polyposis, and Fanconi anemia (3,51). The majority of pediatric HCC is secondary to chronic hepatitis B infection in endemic areas, but implementation of vaccination programs is associated with a decrease of incidence (52).
Finding incidental small or microscopic foci of HCC is not infrequent in explanted cirrhotic livers after transplantation (i.e., the overall risk of developing an HCC in biliary atresia is estimated around 2%) (53). Therefore, specific follow-up program aiming to ensure early detection of malignancy by AFP blood levels and ultrasound imaging are regularly performed in pediatric patients with cirrhosis.
In children with HCC on chronic liver disease, the indication to LT has been well recognized, regardless to tumor size, in order to remove the underlying risk factor of HCC. Initially adult HCC criteria for LT have been proposed for children with HCC on a background of liver disease, however, since at diagnosis many pediatric HCC are outside of Milan criteria, satisfactory results were reported when pediatric LT was performed outside these criteria (8). An Italian experience of 10 HCC children with underlying liver cirrhosis (including 6 patients outside of Milan criteria), who received LT without neoadjuvant chemotherapy, showed that no tumor recurrence was observed after 4 years of follow-up (40). Romano et al. comment that a favorable condition for these good results was related to the short time on the waiting list [38 (range, 1–152) days] due to Italian pediatric organ allocation policy, which provides a priority of liver allocation for HCC on the national pediatric LT waiting list (54). These excellent outcomes suggest that children with HCC should be prioritized on the LT waiting list, since the implementation of split liver programs and living-related LT permit to provide an adequate source of organs for pediatric recipients (55).
In 2017, Vinayak et al. (41) performed a retrospective review of 149 LT for unresectable HCC in children from the US Scientific Registry of Transplant Recipients, observing a patient survival rate of 85% at 1 year, 51.7% at 5 years and 42.7% at 10 years of follow-up; interestingly, patients with incidental HCC on the explanted liver showed superior survival compared to these who underwent LT for primary HCC (85% vs. 48.3% at 10 years of follow-up, respectively). In this report, the outcomes of 25 pediatric LT recipients performed at the Children’s Hospital of Pittsburgh have been detailed, showing that 36% of HCC patients had tumor recurrence after 10 years of follow-up and only vascular invasion was identified as risk factor (P<0.0001).
Recently, Baumann et al. (44) reported the largest cohort (n=175) of pediatric HCC patients treated by LT from the European Liver Transplant Registry (ELTR), comparing LT outcomes of children and adults as well as patients with and without underlying inherited liver disease. The ELTR overall 5-year patient (57.6%) and graft survival rates (56.3%) of HCC children resulted similar to those reported by the UNOS registry on pediatric HCCs (5-year patient survival: 53.5%, 5-year graft survival: 42.8%) (46); a superior long-term survival of children with inherited liver disease was observed not only when compared to HCC children without inherited liver disease (HR =0.29; 95% CI, 0.10–0.90; P=0.03), but also compared to adults affected by HCC associated with inherited liver disease (HR =0.27; 95% CI, 0.06–1.25; P=0.09). As authors suggest, the better results in children with chronic liver disease may be related to the strict follow-up program, which allows early diagnosis of malignancy. Therefore, consistent part of the management of the predisposing conditions to develop HCC is oriented to their early detection and these ELTR data should be taken into consideration for future pediatric transplant organ allocation policy.
Conclusions
HCC is a rare malignancy in children, occurring mainly on a background of normal liver, and its clinical behavior is different from adult HCC. The management of pediatric HCC has evolved in the last decades, and it’s nowadays based on aggressive approaches, including surgery, chemotherapy and loco-regional treatments. This can be only achieved referring patients at diagnosis in highly specialized centers, with pediatric oncology, hepatobiliary surgery and LT programs, and interventional radiology.
Surgery, whether liver resection or LT, is the only treatment that can obtain HCC eradication and recurrence-free survival, however, overall survivals are still unsatisfactory. Until recently, the role of LT in pediatric HCC treatment has been limited, mainly because of organ shortage. Nowadays, split LT and living donor LT offer the opportunity to increase the number of children undergoing LT. The analysis of the available data suggests that, when possible, upfront HCC surgery should be performed, possibly preceded by chemotherapy, in order to evaluate the biological response of the tumor (decrease of AFP) and to minimize the risk of extra hepatic spread of the disease. In case of locally advanced HCC and/or extra-hepatic lesions, chemotherapy and/or loco-regional treatments are indicated in order to downstage the disease. In this setting, the role of sorafenib and other biological agents needs to be further explored. Downstage responders benefit of tumor macro- and microscopic radical (R0) resection, while in case of persistent locally advanced tumor without extra-hepatic localizations, LT with adequate priority on the waiting list should be performed, rather than insisting on chemotherapy. In resectable HCC, whether LT could offer better results than conventional resection needs to be explored.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Meyers RL. Tumors of the liver in children. Surg Oncol 2007;16:195-203. [Crossref] [PubMed]
- Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol 2014;28:753-70. [Crossref] [PubMed]
- Kelly D, Sharif K, Brown RM, et al. Hepatocellular carcinoma in children. Clin Liver Dis 2015;19:433-47. [Crossref] [PubMed]
- Lau CS, Mahendraraj K, Chamberlain RS. Hepatocellular Carcinoma in the Pediatric Population: A Population Based Clinical Outcomes Study Involving 257 Patients from the Surveillance, Epidemiology, and End Result (SEER) Database (1973-2011). HPB Surg 2015;2015. [Crossref] [PubMed]
- López-Terrada D, Alaggio R, deDávila MT, et al. Children’s Oncology Group Liver Tumor Committee. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 2014;27:472-91. [Crossref] [PubMed]
- Katzenstein HM, Krailo MD, Malogolowkin MH, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children’s Cancer Group intergroup study. J Clin Oncol 2002;20:2789-97. [Crossref] [PubMed]
- Meyers RL, Tiao G, de Ville de Goyet J, et al. Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 2014;26:29-36. [Crossref] [PubMed]
- de Ville de Goyet J, Meyers RL, Tiao GT, et al. Beyond the Milan criteria for liver transplantation in children with hepatic tumours. Lancet Gastroenterol Hepatol 2017;2:456-462. [Crossref] [PubMed]
- Pham TA, Gallo AM, Concepcion W, et al. Effect of liver transplant on long-term disease-free survival in children with hepatoblastoma and hepatocellular cancer. JAMA Surg 2015;150:1150-8. [Crossref] [PubMed]
- Pircher A, Medinger M, Drevs J. Liver cancer: Targeted future options. World J Hepatol 2011;3:38-44. [Crossref] [PubMed]
- Prokurat A, Kluge P, Kościesza A, et al. Transitional liver cell tumors (TLCT) in older children and adolescents: a novel group of aggressive hepatic tumors expressing beta-catenin. Med Pediatr Oncol 2002;39:510-8. [Crossref] [PubMed]
- Czauderna P. Adult type vs. Childhood hepatocellular carcinoma--are they the same or different lesions? Biology, natural history, prognosis, and treatment. Med Pediatr Oncol 2002;39:519-23. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13. [Crossref] [PubMed]
- Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after trans-arterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol 2015;63:83. [Crossref] [PubMed]
- Allan BJ, Wang B, Davis JS, et al. A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg 2014;49:166-71; discussion 171. [Crossref] [PubMed]
- Murawski M, Weeda VW, Maibach R, et al. Hepatocellular carcinoma in children: does modified platinum- and doxorubicin-based chemotherapy increase tumor resectability and change outcome? Lessons learned from the SIOPEL 2 and 3 studies. J Clin Oncol 2016;34:1050-6. [Crossref] [PubMed]
- McAteer JP, Goldin AB, Healey PJ, et al. Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatr Transplant 2013;17:744-50. [Crossref] [PubMed]
- Malek MM, Shah SR, Atri P, et al. Review of outcomes of primary liver cancers in children: our institutional experience with resection and transplantation. Surgery 2010;148:778-82. [Crossref] [PubMed]
- Zarrinpar A, Kaldas F, Busuttil RW. Liver transplantation for hepatocellular carcinoma: an update. Hepatobiliary Pancreat Dis Int 2011;10:234-42. [Crossref] [PubMed]
- Austin MT, Leys CM, Feurer ID, et al. Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 2006;41:182-6. [Crossref] [PubMed]
- Wang J, Mao Y, Liu Y, et al. Hepatocellular Carcinoma in Children and Adolescents: Clinical Characteristics and Treatment. J Gastrointest Surg 2017;21:1128-35. [Crossref] [PubMed]
- Lungren MP, Towbin AJ, Roebuck DJ, et al. Role of interventional radiology in managing pediatric liver tumors: Part 1: Endovascular interventions. Pediatr Radiol 2018;48:555-64. [Crossref] [PubMed]
- Czauderna P, Mackinlay G, Perilongo G, et al. Hepatocellular carcinoma in children: Results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol 2002;20:2798-804. [Crossref] [PubMed]
- Hiyama E. Current therapeutic strategies for childhood hepatic malignant tumors. Int J Clin Oncol 2013;18:943-5. [Crossref] [PubMed]
- Chen JC, Chen CC, Chen WJ, et al. Hepatocellular carcinoma in children: Clinical review and comparison with adult cases. J Pediatr Surg 1998;33:1350-4. [Crossref] [PubMed]
- Litten JB, Tomlinson GE. Liver tumors in children. Oncologist 2008;13:812-20. [Crossref] [PubMed]
- Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006;66:11851-8. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Peck-Radosavljevic M, Greten TF, Lammer J, et al. Consensus on the current use of sorafenib for the treatment of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2010;22:391-8. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a Phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Schmid I, Häberle B, Albert MH, et al. Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr Blood Cancer 2012;58:539-44. [Crossref] [PubMed]
- Kaliciński P, Ismail H, Broniszczak D, et al. Non resectable hepatic tumours in children - Role of liver transplantation. Ann Transplant 2008;13:37-41. [PubMed]
- Schmid I, von Schweinitz D. Pediatric hepatocellular carcinoma: challenges and solutions. J Hepatocell Carcinoma 2017;4:15-21. [Crossref] [PubMed]
- Squires RH, Ng V, Romero R, et al. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology 2014;60:362-98. [Crossref] [PubMed]
- Guiteau JJ, Cotton RT, Karpen SJ, et al. Pediatric liver transplantation for primary malignant liver tumors with a focus on hepatic epithelioid hemangioendothelioma: the UNOS experience. Pediatr Transplant 2010;14:326-31. [Crossref] [PubMed]
- Kosola S, Lauronen J, Sairanen H, et al. High survival rates after liver transplantation for hepatoblastoma and hepatocellular carcinoma. Pediatr Transplant 2010;14:646-50. [Crossref] [PubMed]
- Romano F, Stroppa P, Bravi M, et al. Favorable outcome of primary liver transplantation in children with cirrhosis and hepatocellular carcinoma. Pediatr Transplant 2011;15:573-9. [PubMed]
- Vinayak R, Cruz RJ Jr, Ranganathan S, et al. Pediatric liver transplantation for hepatocellular cancer and rare liver malignancies: US multicenter and single-center experience (1981-2015). Liver Transpl 2017;23:1577-88. [Crossref] [PubMed]
- Khan AS, Brecklin B, Vachharajani N, et al. Liver Transplantation for Malignant Primary Pediatric Hepatic Tumors. J Am Coll Surg 2017;225:103-13. [Crossref] [PubMed]
- Kohorst MA, Warad DM, Matsumoto JM, et al. Management of pediatric hepatocellular carcinoma: A multimodal approach. Pediatr Transplant 2017;21. [Crossref] [PubMed]
- Baumann U, Adam R, Duvoux C, et al. Survival of children after liver transplantation for hepatocellular carcinoma. Liver Transpl 2018;24:246-55. [Crossref] [PubMed]
- Hamilton EC, Balogh J, Nguyen DT, et al. Liver transplantation for primary hepatic malignancies of childhood: The UNOS experience. J Pediatr Surg 2017. [Epub ahead of print]. [PubMed]
- Weeda VB, Murawski M, McCabe AJ, et al. Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma: results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer 2013;49:2698-704. [Crossref] [PubMed]
- Manzia TM, Lai Q, Iesari S, et al. Impact of remnant vital tissue after locoregional treatment and liver transplant in hepatocellular cancer patients, a multicentre cohort study. Transpl Int 2018. [Epub ahead of print]. [Crossref] [PubMed]
- PLUTO. Accessed on 1 August 2018. Available online: http://pluto.cineca.org/webdemo/
- Ismail H, Broniszczak D, Kalicinski P, et al. Liver transplantation in children with hepatocellular carcinoma. do Milan criteria apply to pediatric patients? Pediatr Transplant 2009;13:682-92. [Crossref] [PubMed]
- Otte JB. Should the selection of children with hepatocellular carcinoma be based on Milan criteria? Pediatr Transplant 2008;12:1-3. [Crossref] [PubMed]
- Manzia TM, Angelico R, Toti L, et al. Glycogen storage disease type Ia and VI associated with hepatocellular carcinoma: two case reports. Transplant Proc 2011;43:1181-3. [Crossref] [PubMed]
- Chang MH, You SL, Chen CJ, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst 2009;101:1348-55. [Crossref] [PubMed]
- Tatekawa Y, Asonuma K, Uemoto S, et al. Liver transplantation for biliary atresia associated with malignant hepatic tumors. J Pediatr Surg 2001;36:436-9. [Crossref] [PubMed]
- TRAPIANTI. Updated on the 8 August 2018. Available online: http://www.trapianti.salute.gov.it/
- Perito ER, Roll G, Dodge JL, et al. Split liver transplantation and pediatric waitlist mortality in the United States: potential for improvement. Transplantation 2018. [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Angelico R, Grimaldi C, Saffioti MC, Castellano A, Spada M. Hepatocellular carcinoma in children: hepatic resection and liver transplantation. Transl Gastroenterol Hepatol 2018;3:59.


