Pediatric gastrointestinal stromal tumors—a review of diagnostic modalities
Introduction
Gastrointestinal stromal tumors (GIST) are defined as gastrointestinal tumors of mesenchymal origin, specifically the interstitial cells of Cajal, which are considered the pacemakers of the gastrointestinal tract. GIST have a reported incidence of 10 to 15 cases per million overall (1), however the incidence of GIST in the pediatric population is difficult to attain due to their rare nature and these tumors are often misdiagnosed as other acute or chronic abdominal conditions. However, a study in the Lancet in 2013 indicates an annual incidence of 0.02 per million, or about 0.4% of all GIST patients are <20 years old (2) and SEER data estimates from a study in 2015 the incidence of GIST in subjects aged between 8–20 years old to be approximately 0.11 cases per million subjects or 1.64% of all GIST cases. The pediatric GIST patient is a distinct entity and clinicopathologic characteristics vary from the adult form of GIST. Prior to molecular genetic studies, the adult and pediatric GIST forms were separated by age of presentation and histologic features. However, with the advent of genetic testing, it has been discovered that 85% of the pediatric patients with GIST will have a tumor that lacks a mutation in KIT or PDGFRA. Rather, many will contain a loss of function mutation in the succinate-dehydrogenase complex (3). Tumors lacking the KIT/PDGFRA mutation are also known as “wild-type” (WT) GIST.
Clinical behavior
Pediatric/WT GIST behaves in a much different fashion clinically than the adult variant. Age of onset for the Pediatric/WT GIST is much younger with a median in the second decade of life (3), whereas the adult variant median age of diagnosis is 63. There is a female preponderance in the pediatric population for development of GIST (70% of diagnoses), while the adult GIST population has equal gender distribution. Clinically, a pediatric GIST is most likely to present as a GI bleed, likely due to its predilection for the stomach in pediatric patients. Other symptoms include: abdominal complaints (pain, appetite change, early satiety, bloating, vomiting, constipation, or obstipation), weight loss, and anemia. As mentioned previously, pediatric GIST are exceedingly rare and often go undiagnosed or misdiagnosed. The distribution of location of primary tumor is not as broad as the adult GIST, as the pediatric GIST tumors predominate in the stomach, with some rarely located in the small bowel and other gastrointestinal locations (4). Pediatric/WT GIST is also much more likely (45% at diagnosis) to involve lymph nodes, liver, or the peritoneum. The most striking difference in the Pediatric/WT GIST tumor population, however, is that the tumors tend to have an indolent course, meaning that many of the patients will live with their tumors for years (5). It is important to consider this when determining treatment regimens for pediatric patients and their potential long lifespan.
Cancer syndromes
Pediatric/WT GIST in addition to occurring sporadically, are also associated with cancer syndromes. Carney’s triad which includes GIST, pulmonary chondroma, paraganglioma, was first described by Carney in 1977 (6). This results from a non-heritable loss of expression mutation in the SDH-B complex. The second syndrome is the Carney-Stratakis syndrome which includes GIST and paraganglioma. This is a heritable, autosomal-dominant syndrome with a germline inactivating mutation in SDH (subunit A,B,C,D) (7). The third syndrome associated with GIST is Von Recklinghausen’s disease, or neurofibromatosis 1 (NF1). This syndrome is characterized by cafe au lait spots, lisch nodules, freckling, and neurofibromas, however GIST are the most common non-neurological malignancy in this particular patient population, occurring in about 7% of patients with NF1 (8).
Molecular characteristics
Over the last two decades, our understanding of the genetic mutations involved in the development of GIST has grown exponentially, so much that some are proposing a new classification scheme for GIST that comprises either SDH-competent (adult GIST or KIT/PDGFRA mutation) and SDH-deficient (Pediatric/WT GIST) tumors. The SDH-competent GIST category includes those tumors that have mutations in either KIT (75% of all tumors), PDGFRA (~5–10% of tumors), BRAF [~5% (9)], and other more rare mutations. The SDH-deficient tumors are a rarer form. Boikos et al. in 2016 showed that around 88% of GIST that had been classified as Pediatric/WT were shown to be SDH-complex deficient upon genetic evaluation (3). These tumors occur almost exclusively in the stomach (10), manifest at younger ages, are more likely to occur in female patients, and show early lymphovascular invasion. Chou et al. reported that these tumors also show overexpression of insulin-like growth factor 1 (IGFR-1) which, when activated, can also manifest neuroendocrine pathologies such as paragangliomas, adrenal tumors, and adrenal nodules (11). Due to the high concordance with molecular characteristics and the clinical manifestations and course, it is highly recommended that every GIST undergo a thorough evaluation of the mutations involved as this will guide management decisions.
Diagnosis
The diagnosis of GIST in the pediatric population can be clinically challenging due to the vague indolent symptoms, in addition to the rare nature of this disease. Imaging remains an important modality for diagnosis and determining anatomic relationships, however concerns for radiation exposure in this population must be considered when choosing an imaging modality. The presenting symptomatology of the patient however, may dictate the type of imaging modality utilized initially.
Computed tomography (CT)
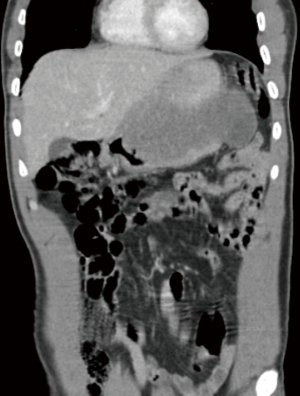
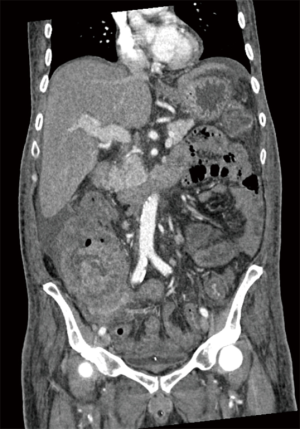
In the adult population, CT scan is the gold standard imaging modality for diagnosis and continued follow-up (12-14). However, radiation exposure is a concern in the pediatric population (Table 1). Depending on the child’s weight and CT protocol used, the exposure ranges from 5-11 mSV (15). Newer 3rd generation CT scanners with iterative reconstruction have been shown to decrease this dosage by about 33% (16). Even still, CT scan is utilized with caution in the pediatric population and although not routinely utilized for surveillance due to the radiation exposure over-time, may be utilized upon presentation depending on acuity of symptoms (Table 1). CT characteristics for GIST vary depending on size and primary vs. metastatic disease. A large (>5 cm) lesion is typically exophytic, hypervascular with heterogeneous enhancement on contrast enhanced CT (17) (Figures 1,2), while the smaller lesions (<5 cm) are typically submucosal or endoluminal polypoid masses that show homogenous contrast enhancement (18). Metastasis on CT will have arterial enhancement (due to its hypervascular nature) and will lack enhancement on portal venous and venous phases (19) (Table 1).

Full table
Magnetic resonance imaging (MRI)
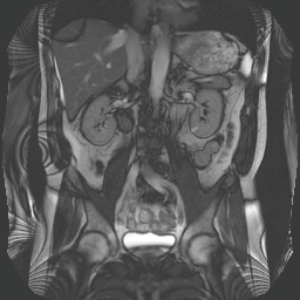
MRI modality of the abdomen/pelvis is the preferred method for diagnosis, surgical planning, and surveillance due to its ability to define the lesion with respect to the abdominal cavity and its lack of ionizing radiation (17) (Table 1). It is recommended that the German GIST Imaging Working Group protocol be utilized for these cases (14). According to a paper by Herzberg et al. in 2018, it is recommended to utilize MRI, contrast-enhanced ultrasound (CEUS), and positron-emission tomography (PET)-CT/MR (depending on availability) for initial staging with surveillance imaging with MRI and CEUS every 6 months for at least 3 years post-therapy, with the caveat that lifelong annual imaging may be required due to the chronicity of this disease (17). Features of GIST on MRI are also variable depending on size and presence of high-risk tumor features. Large lesions (>5 cm) are typically irregular, lobulated, contain mild, gradual, and heterogeneous contrast enhancement, and often have intratumoral cysts (Figure 3), while the small tumors (<5 cm) show intense homogenous enhancement in the arterial contrast phase (17) (Table 1). MRI has been shown to aid in the adult population with prediction of high-risk tumors; those that contain low apparent diffusion coefficient (ADC) and presence of intratumoral cysts (20). However, this benefit has not been studied in the pediatric population.
Contrast enhanced ultrasound (CEUS)
CEUS combines ultrasonography with a contrast agent that acts as a pure blood pool agent, allowing for the evaluation of hypovascular and hypervascular lesions as small as 40 micrometers (21) (Table 1). As stated previously, the guidelines suggested by Herzberg et al. utilize CEUS of the tumor and liver in both the initial staging process and subsequent serial surveillance imaging (17). In addition to its utility in staging/surveillance scenarios, CEUS has also been shown efficacious to assess disease progression and treatment response when tyrosine kinase inhibitors (TKIs) are utilized (Table 1). It is thought that treatment alters tumor structure, causes decreased vascularity and eventual tumor necrosis, which CEUS can detect (22) and has increased sensitivity over PET scan for small hypovascular lesions (23). The characteristics of GIST on CEUS are hypervascular on arterial phase and hypoenhancing on portal venous phase (17). Dietrich describes low-risk GISTs to be homogenously enhancing, while high-risk GISTs tend to show more heterogeneous enhancement with areas of avascularity (24) (Table 1). CEUS does not have the ability to differentiate GIST from other lesions such as leiomyomas and schwannomas, however one study demonstrated an ADC increase of >30% in GIST imaging after imatinib treatment was administered (25).
Positron-emission tomography (PET)
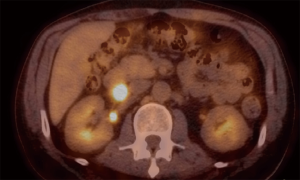
PET scans (either CT/MR depending on availability) have high sensitivity (90–100%) for the detection of metastasis and can be utilized for early response assessment when using TKI therapy (14,26,27). However with cost-utilization taken into consideration, CEUS has similar sensitivity when anatomy allows its use and should be utilized if possible before PET scan is employed for surveillance (26). As stated previously, it is recommended to use PET-MR (if available, CT if not available) for initial staging in the pediatric population (17,28). Due to its high avidity for brown adipose tissue, and the pediatric population’s higher percentage of this type of fat, it is recommended to pre-treat patients with a beta-blocker which can aid reducing the [18] fludeoxyglucose (FDG) avidity for brown adipose tissue and will decrease the false-positive rate. PET characteristics of GIST will show a homogenous increase in tracer due to increased glycolytic activity of the tumor (Figure 4) (Table 1).
Endoscopy
Endoscopy is typically utilized in the pediatric population when there is a suspicion for either an obstructing mass, or more commonly, a gastrointestinal bleed, and this is often how GIST presents in the pediatric population. A GIST lesion will tend to appear as a submucosal mass with smooth margins that causes protrusion of overlying mucosa, with occasional associated necrosis or hemorrhage (29). Concurrent utilization of endoscopic ultrasound (EUS) at the time of exam allows for distinction between intramural and extramural lesions and may guide tissue sampling (30). A recent article by Chhoda et al. in 2018 in the adult population demonstrated that contrast-enhanced endoscopic ultrasound is a promising modality to detect perfusion patterns and demonstrates higher sensitivity than EUS-fine-needle aspiration (FNA), contrast CT, and Doppler EUS for detection of neovascularity within a lesion (31). Although neovascularity as a prognostic factor within a GIST needs to be further evaluated with prospective data, this newer modality elicits a promising new avenue for the diagnosis and treatment surveillance for GIST.
Biopsy
The multiple modalities available for tissue sampling coupled with patient presentation makes obtaining a biopsy for lesions a multidisciplinary approach. The amount of tissue required to make the GIST diagnosis is 1 cubic-centimeter (about 5–10 core-needle biopsy specimens). Endoscopy is the preferred method [core needle biopsy (CNB) or forceps biopsy (FB)] with or without ultrasound guidance (32) due to concerns for tumor spillage via other routes such as percutaneous image-guided biopsy or surgical biopsy. Tumor spillage is highly associated with recurrence in pediatric GIST, and thus should be avoided at all costs (5) Therefore, if anatomy allows and endoscopic biopsy approach is not feasible, it may be prudent to perform primary resection as a means for tissue diagnosis. Percutaneous image-guided techniques are not typically utilized for primary lesions (due to concern of tumor spillage), however this modality might be the preferred approach for sampling of metastatic lesions (5).
Pathology
GIST tumors exhibit three different morphologies: Spindle cell, epithelioid cell, and mixed cell types. Spindle-cell morphology is highly associated with the classic (KIT mutated) adult GIST, whereas the epithelioid and mixed cell morphologies are more highly associated with the Pediatric/WT GIST tumors. The CD117 (KIT) and anoctamin (ANO1) immunohistochemical (IHC) markers are the most sensitive and specific markers for analysis for GIST lesions, however these cannot be utilized in place of mutational analysis (33). In certain pediatric-WT GISTs (SDH deficient) IGF1R via IHC will be positive (11,34). There are currently two risk score estimates developed by the NIH (35) and the Armed Forces Institute of Pathology (AFIP) (36). These risks scores were developed to determine the risk of tumors having aggressive behavior. The NIH criteria incorporates metastatic disease, tumor size, and mitotic count and stratifies tumors into very low risk, low risk, intermediate risk, high risk, and metastatic disease categories (35). The AFIP criteria incorporate tumor size, mitotic count, and primary tumor site (gastric vs. small intestine) and stratifies tumors into very low risk, low risk, intermediate risk, and high risk categories (36). Interestingly, a recent study by Weldon et al. regarding pediatric/WT GIST surgical management demonstrated that postoperatively an increased tumor mitotic rate and elevated NIH risk score were significantly associated with progression or recurrence of disease while the AFIP was not (37).
Conclusions
Pediatric/WT GIST is an extremely rare condition. It is recommended that pediatric patients with GIST be offered referral to specialized centers that can offer a multidisciplinary approach with coordinated, highly specialized care. The initial diagnosis of GIST for pediatric patients should be coordinated with radiology, pathology, gastroenterology, and surgical teams to ensure swift diagnosis and adherence to the most updated clinical guidelines. We suggest a diagnostic algorithm (Figure 5) dependent upon the patient’s clinical presentation. Patients with acute presentations such as obstruction, hemorrhage, or perforation should be quickly evaluated with readily available CT scan and endoscopy. In contrast, patients with a chronic presentation will benefit from a more thorough diagnostic and staging work up. This algorithm is not all encompassing but rather a guideline to aid clinicians that are unfamiliar with gastrointestinal tumors in the pediatric population.

Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39-46. [Crossref] [PubMed]
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973-83. [Crossref] [PubMed]
- Boikos SA, Pappo AS, Killian JK, et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2016;2:922-8. [Crossref] [PubMed]
- Harlan LC, Eisenstein J, Russell MC, et al. Gastrointestinal stromal tumors: treatment patterns of a population-based sample. J Surg Oncol 2015;111:702-7. [Crossref] [PubMed]
- Mullassery D, Weldon CB. Pediatric/"Wildtype" gastrointestinal stromal tumors. Semin Pediatr Surg 2016;25:305-10. [Crossref] [PubMed]
- Carney JA, Sheps SG, Go VL, et al. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med 1977;296:1517-8. [Crossref] [PubMed]
- Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet 2002;108:132-9. [Crossref] [PubMed]
- Miettinen M, Fetsch JF, Sobin LH, et al. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol 2006;30:90-6. [Crossref] [PubMed]
- Agaram NP, Laquaglia MP, Ustun B, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res 2008;14:3204-15. [Crossref] [PubMed]
- Miettinen M, Wang ZF, Sarlomo-Rikala M, et al. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol 2011;35:1712-21. [Crossref] [PubMed]
- Chou A, Chen J, Clarkson A, et al. Succinate dehydrogenase-deficient GISTs are characterized by IGF1R overexpression. Mod Pathol 2012;25:1307-13. [Crossref] [PubMed]
- ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii49-55. [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. [Crossref] [PubMed]
- Kalkmann J, Zeile M, Antoch G, et al. Consensus report on the radiological management of patients with gastrointestinal stromal tumours (GIST): recommendations of the German GIST Imaging Working Group. Cancer Imaging 2012;12:126-35. Erratum in: Cancer Imaging 2013;13:196. [Crossref] [PubMed]
- Kharbanda AB, Krause E, Lu Y, et al. Analysis of radiation dose to pediatric patients during computed tomography examinations. Acad Emerg Med 2015;22:670-5. [Crossref] [PubMed]
- Rompel O, Glöckler M, Janka R, et al. Third-generation dual-source 70-kVp chest CT angiography with advanced iterative reconstruction in young children: image quality and radiation dose reduction. Pediatr Radiol 2016;46:462-72. [Crossref] [PubMed]
- Herzberg M, Beer M, Anupindi S, et al. Imaging pediatric gastrointestinal stromal tumor (GIST). J Pediatr Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Antoch G, Herrmann K, Heusner TA, et al. Bildegebene Verfahren bei gastrointestinalen stromatumoren. Radiologe 2009;49:1109-16. [Crossref] [PubMed]
- Sandrasegaran K, Rajesh A, Rushing DA, et al. Gastrointestinal stromal tumors: CT and MRI findings. Eur Radiol 2005;15:1407-14. [Crossref] [PubMed]
- Charles-Edwards EM, deSouza NM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging 2006;6:135-43. [Crossref] [PubMed]
- Dietrich C, Hartung E, Ignee A. The use of contrast-enhanced ultrasound in patients with GIST metastases that are negative in CT and PET. Ultraschall Med 2008;29 Suppl 5:276-7. [Crossref] [PubMed]
- Lassau N, Lamuraglia M, Chami L, et al. Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol 2006;187:1267-73. [Crossref] [PubMed]
- Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012;33:33-59. [Crossref] [PubMed]
- Dietrich CF, Jenssen C, Hocke M, et al. Imaging of gastrointestinal stromal tumours with modern ultrasound techniques - a pictorial essay. Z Gastroenterol 2012;50:457-67. [Crossref] [PubMed]
- Revheim ME, Hole KH, Bruland OS, et al. Multimodal functional imaging for early response assessment in GIST patients treated with imatinib. Acta Oncol 2014;53:143-8. [Crossref] [PubMed]
- Gayed I, Vu T, Iyer R, et al. The roe of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med 2004;45:17-21. [PubMed]
- Prior JO, Montemurro M, Orcurto MV, et al. Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J Clin Oncol 2009;27:439-45. [Crossref] [PubMed]
- Gatidis S, Schmidt H, Gücke B, et al. Comprehensive Oncologic Imaging in Infants and Preschool Children With Substantially Reduced Radiation Exposure Using Combined Simultaneous 18F-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Imaging: A Direct Comparison to 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. Invest Radiol 2016;51:7-14. [Crossref] [PubMed]
- Papanikolaou IS, Triantafyllou K, Kourikou A, et al. Endoscopic ultrasonography for gastric submucosal lesions. World J Gastrointest Endosc 2011;3:86-94. [Crossref] [PubMed]
- Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc 2002;55:37-43. [Crossref] [PubMed]
- Chhoda A, Jain D, Surabhi VR, et al. Contrast Enhanced Harmonic Endoscopic Ultrasound: A Novel Approach for Diagnosis and Management of Gastrointestinal Stromal Tumors. Clin Endosc 2018;51:215-21. [Crossref] [PubMed]
- Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006;130:2217-28. [Crossref] [PubMed]
- Novelli M, Rossi S, Rodriguez-Justo M, et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology 2010;57:259-70. [Crossref] [PubMed]
- Nannini M, Biasco G, Astolfi A, et al. An overview on molecular biology of KIT/PDGFRA wild type (WT) gastrointestinal stromal tumours (GIST). J Med Genet 2013;50:653-61. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [PubMed]
- Weldon CB, Madenci AL, Boikos SA, et al. Surgical Management of Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Pediatric and Wildtype GIST Clinic. J Clin Oncol 2017;35:523-8. [Crossref] [PubMed]
Cite this article as: Quiroz HJ, Willobee BA, Sussman MS, Fox BR, Thorson CM, Sola JE, Perez EA. Pediatric gastrointestinal stromal tumors—a review of diagnostic modalities. Transl Gastroenterol Hepatol 2018;3:54.


