Laparoscopic distal pancreatectomy: better than open?
Introduction
Laparoscopic distal (left) pancreatectomy (LDP) was performed by expert laparoscopic surgeons as early as 1994 (1,2), but the technique took some time to catch on in overall practice. As for other procedures performed laparoscopically (cholecystectomy, colonic disease, hysterectomy), the minimal access approach is likely to reduce pain, decrease blood loss, shorten hospital stay, enhance the postoperative course, and provide better cosmesis and reduced costs (3,4). The laparoscopic approach is well suited to DP because the visual magnification obtained with high definition cameras under laparoscopy lead to more precise and controlled tissue manipulation and facilitates access to the deep posterior aspect of the gland; moreover, there is no need for reconstruction (5).
While LDP should theoretically provide the same postoperative recovery advantages reputed to minimal access surgery, there have been fears as to the safety of LDP in terms of life-threatening intra-operative events and post-operative complications, adequate carcinological outcomes as compared to traditional (open) distal pancreatectomy (ODP) when performed for cancer (6), as well as to whether the laparoscopic approach is well adapted to the variety of diseases that may affect the pancreas (ranging from trauma to benign or malignant disease) and whether the minimal access approach is well adapted to perform pancreatic surgery safely in the obese, the elderly or the frail.
Our goal was to review the literature to determine whether LDP was as safe, provided the same oncological outcomes and was applicable to all diseases involving the body and tail of the pancreas, and amenable to particular patient characteristics, compared to the traditional open approach. Last we looked at cost issues.
Material and methods
We conducted our systematic review of the literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (7). EMBASE, Medline, Web of science, Cochrane CENTRAL and Google scholar databases were systematically searched up to March 2018 for articles comparing LDP to ODP using the following terms: laparoscopic distal pancreatectomy, distal pancreatectomy, laparoscopic left pancreatectomy, left pancreatectomy, comparison, pancreas left resection, distal pancreas resection; distal pancreatectomy; left pancreatectomy, laparoscopic surgery, minimally invasive surgery; open surgery; laparotomy. We limited our search to manuscripts written in English.
Results
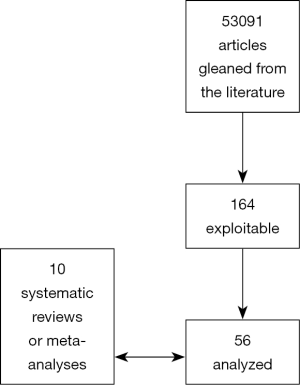
The initial database search resulted in 53,091 articles. After eliminating publications that could have contained duplicate series or duplicates that arose from searching several data bases, 164 potentially relevant articles were screened for appropriateness. Only studies explicitly comparing laparoscopic to open resection were retained. No case series reports were considered.
This resulted in 56 studies available for the systematic review. The PRISMA flowchart presented in Figure 1 depicts the detailed selection of studies.
Among the 56 studies published in full, none were randomized. There were ten meta-analyses or systematic reviews (8-17) including one Cochrane review (18) with considerable overlap. Because of this overlap, we chose to base the main points of our analysis on the four most recent and/or with the least overlap (10,13,15,18). Three concerned only malignancy (13,14,18).
There are currently six randomized trials underway. To the best of our knowledge, two have been completed (LAPOP and LEOPARD) but only one with available results (not published), the LEOPARD study (de Rooij) (19,20).
The proportion of DP performed via minimal access surgery (MAS) is on the rise, accounting for between 10.8% to 46.6% of DP published worldwide (10,21,22). Responders of a worldwide survey including 435 surgeons from 50 countries reported that they performed a median of 22 (IQR: 0–450) pancreatic resections as primary surgeon yearly (23). Minimal access distal pancreatectomy (MADP) was performed by 345 (79%) of these surgeons. Of these surgeons, 338 (98%) considered the overall value of such resections superior or equivalent to the traditional approach.
Results of the analysis
Indications for laparoscopic distal pancreatectomy
The indications for LDP are similar to those for open distal pancreatectomy (10,24-26): distal pancreatectomy for benign, borderline, or malignant tumors of the pancreatic body and tail, distal pancreatectomy for pancreatic injury and chronic or acute pancreatitis with pseudocyst located in the pancreatic body and tail. Obvious contraindications for LDP include: invasion of surrounding organs or critical vasculature, distant metastasis in cancer (22), or acute pancreatitis (27). Obesity is not a formal contra-indication although it makes the operation more challenging (15,24).
Spleen preserving LDP has been described to be feasible and safe (26,28), being reported in 18.2% (29) to 60.4% (30) of LDPs. Spleen preserving techniques may be easier to perform laparoscopically but the preservation of the short gastric vessels, as required in the Warshaw technique (31), might be more complex to perform laparoscopically (32). As well, radical cancer operations require splenectomy, which can be performed laparoscopically, but this will be discussed later.
Operative times
Several comparative publications reporting on duration of operation have found that there was no statistically significant difference between LDP and ODP, ranging from 156 to 383 min and from 145 to 330 min in laparoscopic and open surgery, respectively (16,29,33,34). However this was not analyzed in the recent Cochrane review of 2016 (18).
Conversion
The conversion rate from laparoscopic to open surgery, collated by Justin et al., varied widely from one series to another 0% to 34% (33-37), hemorrhage and failure to progress being the most common causes.
Morbidity and mortality
Complications after LDP have been reported to range between 0 and 67% in single center studies (28,38). However, several meta-analyses (10,35) and systematic reviews (18) have described overall morbidity ranging from 34% to 37.4%.
In their 2015 meta-analysis of 34 studies (10), Mehrabi et al. found a statistically significant difference in time to oral intake (−1.3 days) and duration of stay (−3.8 d). Of note, post-operative stay after DP has been reported to be shorter in the United States when compared to other countries, most likely due to differences in health care systems (35). More recently, Shin and colleagues (22) confirmed these significant reductions associated with LDP single center, propensity matched analysis.
While Riviere et al. (18) found a 2.43 days shorter hospital stay for LDP (mean difference −2.43 days, 95% CI, −3.13 to −1.73; 1,068 participants; five studies; I2=0%), quality of life, time to return to normal activity and time to return to work or blood transfusion requirements were not reported.
The recently finished LEOPARD study (20) (51 LDP vs. 56 ODP in 17 Dutch centers, operated on for symptomatic benign or malignant tumors of the body or tail) found that LDP was statistically significantly associated with less blood loss (150 vs. 400 mL, P<0.001), longer operation time (217 vs. 179 min) (P=0.05), shorter duration of hospital stay {6 [4–7] vs. 8 [6–9] days} [median (IQR)] (P<0.001) and faster functional recovery {4 [3–6] vs. 6 [5–8]} (P<0.001) (defined as patients being independently mobile, adequately controlled for pain, maintaining at least 50 of daily required intake, no need for intravenous administration and no signs of infection). No statistically significant difference was found in morbidity (Dindo/Clavien grade 3 or higher) [13 (25%) vs. 21 (38%)], POPF grades B/C [20 (39%) vs. 13 (23%)] or 90-day mortality [0 (0%) vs. 1 (2%)] (20).
Post-operative pancreatic fistula (POPF)
Morbidity is essentially related to post-operative pancreatic fistula (POPF), wound infection and omental infarction (27). This might explain why there is a wide range of morbidity rates because authors have used many different definitions for POPF in their literature. Adhering to the ISGPF definition, systematic reviews have described the POPF incidence to range from 16.8% to 21.7% in LDP (10-25).
One systematic review and meta-analysis (17) reported that LDP (compared to open, all other factors being equal) was associated with less POPF. This contrasts with the results of the studies by Sa Cunha et al. (32) and Cho et al. (39), who found that postoperative complications (including POPF) were similar in the two groups. Of note, the meta-analysis (17) included only observational, and heterogeneous studies, while the Central Pancreas Consortium Study (39) encompassed only expert centers, a potential selection bias.
Røsok et al. (15) (16 studies) did not find any statistically significant difference in the rates of B/C POPF. Of note, they included three studies that were not included in any of the other meta-analyses (38,40,41).
The Cochrane production on distal pancreatectomy for cancer only (18) analyzed the outcomes of 11 studies (1,506 participants: 353 undergoing laparoscopic distal pancreatectomy and 1,153 undergoing open distal pancreatectomy) providing data for one or more outcomes. The authors were unable to come to any conclusions concerning the differences in short-term or long-term mortality, the proportion of patients with serious or any adverse events, or with a clinically relevant POPF.
Hand-sewn or stapled closures are the most common techniques used for pancreatic remnant management after LDP. While the systematic review of observational studies (42) was in favor of stapled closure, Jensen et al. (43) [analysis of 10 studies (five retrospective reviews, five prospective case series) between 2007 and 2009] found the overall relative risk of developing a POPF following distal pancreatectomy was 1.00 (95% CI, 0.65–1.53). Although no conclusions could be formally proposed, the authors suggested that reinforced staples could be an attractive solution as when only prospective studies were analyzed, the RR was 14.45 (95% CI, 3.15–66.21). Overall, the meta-analysis of Wang et al. (44), two high-volume institutional studies (45,46) and two RCT (42,47) found that stapled stump closure was associated with a slightly higher POPF rate. Two meta-analyses (48,49), one high-volume study (50) and one multicenter (21 European centers) randomized controlled trial (42), however, were not able to show any statistically significant differences in POPF or overall morbidity between the two closure techniques, whether performed by laparoscopy or laparotomy. Of note, several of these studies (42,45,47,48) did not distinguish between clinically silent and clinically relevant POPF. In the Sa Cuhna study on the usefulness of TachoSil to cover the pancreatic stump after division (32), hand-sewn closure of the pancreatic stump and ligation of the splenic vessels in case of splenic preservation were found to have a significant risk for clinically relevant POPF; this is of interest in the modern era where more and more distal pancreatectomies are being performed laparoscopically (46,51) and stapled stump closure and splenic vessel preservation are preferred in laparoscopic distal pancreatectomy (26,27,32).
One of the reasons for the discrepancy of outcome might be the effect of crushing the pancreatic parenchyma by the jaws of the linear stapler. Effectively, when the pancreas is thick (>12 mm), staple closure may in fact increase the POPF rate (52). As recommended in general, gradual closing of the stapler jaws (2–3 minutes) has been reported to decrease POPF (53). More recently, several authors have insisted on the necessity to first gradually crush the future line of resection before actually firing the stapler (54,55).
Older studies reported that reoperation rates (ranging from 2.1% to 6%) (17,35,56) after laparoscopic DP did not differ from outcomes after open surgery.
Several meta-analyses have found that LDP, even with splenectomy, was superior to PDP in terms of intraoperative blood loss and duration of hospital stay (10,16,17) confirming the results of several controlled trials (21,33,57,58) in this sense.
Carcinological concerns
Several experienced (5-28) laparoscopic surgeons have reported (29-50) that it is easier to achieve an R0 resection with the laparoscopic approach (50-61) while others argue that there is no significant difference in R0 resection rates between laparoscopic or open DP (21,62-64).
Radical operations such as the radical antegrade modular pancreatosplenectomy
RAMP procedure (65), the clockwise procedure (53), are technically challenging laparoscopically, but do appear to be feasible (66,67).
Both the systematic review by Ricci et al. (14) and Mehrabi et al. (10) confirmed that there was no statistically significant difference in R0 resection rates, lymph node harvest and survival, although they did not include the same studies (five and four studies respectively).
The systematic review by Ricci et al. (14) including five studies (58,62,64,68,69) found that R0 resection margins were 86.3% vs. 80.7% with an OR of 1.29 (95% CI, 0.59 to 2.82; P=0.53), lymph node retrieval (14.4±7.3, 13.3±7.6), and survival (HR of 0.66; 95% CI, 0.29–1.51; P=0.32) between the two groups. The meta-analysis by Mehrabi et al. (10) including four studies (63,70-72), reported comparable R0 rates in both groups (592 patients) (OR 1.63; 95% CI, 0.65–4.07; P=0.29), while the rate of R1 resections was lower in the LDP group (520 patients) (OR 0.34; 95% CI, 0.14–0.83; P=0.02) (70,71). None of the studies included in the Riviere Cochrane review reported on recurrence within six months (18).
According to the International Study Group on Pancreatic Surgery guidelines (73), lymph nodes in stations 10, 11, and 18 should be resected during distal pancreatectomy for cancer. Resection of lymph nodes in stations 8a and 9 is optional, but should be included in the resection when cancer is located in the body of the pancreas.
Lee et al. (58) reported that the lymph node yield was higher in open surgery as compared to laparoscopic (or robotic) distal pancreatectomy. This contrasts with the other oncological outcomes in this same comparison.
In the review of Wang et al. (44), there was no significant difference found in the mean number of lymph nodes harvested between LDP and ODP (12 to 13.8 in LDP vs. 10 to 12.5 in ODP) (21,22). However, the median number (10, range 1–64) of lymph nodes harvested in the ODP group in one report (22) was less than 12, the recommended number for adequate disease staging (74).
Shin et al. reported a median survival of 33.4 months in LDP vs. 29.1 months in OPD (P=0.025) (22). While a multicenter study by Kooby et al. showed considerably shorter survival (16 months) in both groups (21). While low long-term survival rates are typical for pancreatic cancer, the difference in survival between these last two studies could be related to the differences in median tumor diameter (3.0 vs. 3.5 cm) and/or the type of (monocenter vs. multicenter) study.
In the Rivière Cochrane review (18), the authors were also unable to come to any formal conclusions concerning the differences in recurrence at maximal follow-up or the proportion of participants with positive resection margins. Thus, the oncological results of LDP in patients with pancreatic ductal adenocarcinoma is unclear. Of note, the lymph node status is an oncologically important variable, as pancreatic ductal adenocarcinoma of the pancreatic body and tail frequently metastasize to regional lymph nodes. In cases of resectable pancreatic ductal adenocarcinoma located in the body and tail, positive lymph nodes are found in 47–80% of resected specimens (75). In the previous report on LDP for exocrine carcinoma from one expert center, a median of 5 [0–26] lymph nodes were found in the specimen; notwithstanding, the 3-year survival was still relatively high (30%) (60). Although a recent meta-analysis of distal pancreatectomy for pancreatic ductal adenocarcinoma suggested that lymph node yields were comparable following laparoscopic or open DP, the small number of cases in laparoscopic group (n=80) limits the reliability of these findings (14).
Performing LDP in patients with obesity was raised as a specific concern in the Minimal Invasive Surgery symposium held in Sao Paulo April 2016 (15). Studies on the impact of obesity on laparoscopic pancreatic resections are few. Of note, in the retrospective cohort study of elective open versus minimal access distal pancreatectomy using the American College of Surgeons’ National Quality Improvement Program (ACS-NSQIP) database (106 centers in 2014), only 8% of the respondents considered morbid obesity as a contra-indication for minimal access DP; however, milder forms of obesity were not (15,20,23). In a Dutch nationwide study comparing LDP to ODP, increased BMI was not associated with higher complication rates in the laparoscopic group (40). A Norwegian single high volume center study (76) found that obesity (BMI >30) led to longer operative duration and increased blood loss compared to patients who were overweight (BMI between 25–30) and those whose BMI was 25 or less. There was no statistically significant difference found in duration of hospital stay or complication rates. As noted above, the expert panel agreed that higher BMI could lead to specific challenges, but high BMI alone was not a contra-indication and did not preclude an attempt to perform a LDP.
The same Norwegian group also studied the outcomes of LDP in the elderly and found that LDP was safe in patients aged ≥70 years, providing outcomes similar to those in younger group (77). In a French multicenter study (78), the laparoscopic approach was associated with reduced blood loss, postoperative confusion, and duration of stay in elderly patients requiring distal pancreatectomy. Overall, these two studies confirm that elderly patients with pancreatic adenocarcinoma can benefit from LDP, since age itself is not associated with decreased survival after surgery.
Costs
Costs were evaluated in a review article by Conlon and the Minimally Invasive Pancreatic Resection Organizing Committee and presented in the International Hepatobiliary and Pancreatic meeting in Sao Paulo on April 20, 2017 (79). Minimally invasive pancreatic resections: cost and value perspectives. Compared to the open approach, minimal access distal pancreatic resections were associated with higher operative costs but lower postoperative costs. Patient-related values (defined as improvement in both quantity and quality of life) and financial value (using incremental cost-effectiveness ratio) remain to be determined.
In conclusion, this review of the literature allows us to state that laparoscopic distal pancreatectomy is feasible and safe for a wide range of diseases, both benign and malignant. Morbidity, mortality, and probably, also, carcinological outcomes are comparable to open surgery. The advantages of minimal access surgery should lead to propose this approach preferentially, once the surgical expertise is acquired and present.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery 1996;120:1051-4. [Crossref] [PubMed]
- Cuschieri A, Jakimowicz JJ, van Spreeuwel J. Laparoscopic distal 70% pancreatectomy and splenectomy for chronic pancreatitis. Ann Surg 1996;223:280-5. [Crossref] [PubMed]
- Sain AH. Laparoscopic cholecystectomy is the current ‘gold standard’ for the treatment of gallstone disease. Ann Surg 1996;224:689-90. [Crossref] [PubMed]
- Schwenk W, Haase O, Neudecker J, Muller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev 2005. [PubMed]
- Stauffer JA, Asbun HJ. Minimally Invasive Pancreatic Surgery. Semin Oncol 2015;42:123-33. [Crossref] [PubMed]
- Bauman MD, Becerra DG, Kilbane EM, et al. Laparoscopic distal pancreatectomy for pancreatic cancer is safe and effective Surg Endosc 2018;32:53-61. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med 2009;151:264-9. [Crossref] [PubMed]
- Jin T, Altaf K, Xiong JJ, et al. A systematic review and meta-analysis of studies comparing laparoscopic and open distal pancreatectomy. HPB 2012;14:711-24. [Crossref] [PubMed]
- Jusoh AC, Ammori BJ. Laparoscopic versus open distal pancreatectomy: a systematic review of comparative studies. Surg Endosc 2012;26:904-13. [Crossref] [PubMed]
- Mehrabi A, Hafezi M, Arvin J, et al. A systematic review and meta-analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: It's time to randomize. Surgery 2015;157:45-55. [Crossref] [PubMed]
- Nigri GR, Rosman AS, Petrucciani N, et al. Metaanalysis of trials comparing minimally invasive and open distal pancreatectomies. Surg Endosc 2011;25:1642-51. [Crossref] [PubMed]
- Pericleous S, Middleton N, McKay SC, et al. Systematic Review and Meta-Analysis of Case-Matched Studies Comparing Open and Laparoscopic Distal Pancreatectomy Is It a Safe Procedure? Pancreas 2012;41:993-1000. [Crossref] [PubMed]
- Postlewait LM, Kooby DA. Laparoscopic distal pancreatectomy for adenocarcinoma: safe and reasonable? J Gastrointest Oncol 2015;6:406-17. [PubMed]
- Ricci C, Casadei R, Taffurelli G, et al. Laparoscopic versus open distal pancreatectomy for ductal adenocarcinoma: a systematic review and meta-analysis. J Gastrointest Surg 2015;19:770-81. [Crossref] [PubMed]
- Røsok BI, de Rooij T, van Hilst J, et al. Minimally invasive distal pancreatectomy. HPB 2017;19:205-14. [Crossref] [PubMed]
- Sui CJ, Li B, Yang JM, et al. Laparoscopic versus open distal pancreatectomy: a meta-analysis. Asian J Surg 2012;35:1-8. [Crossref] [PubMed]
- Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. [Crossref] [PubMed]
- Riviere D, Gurusamy KS, Kooby DA, et al. Laparoscopic versus open distal pancreatectomy for pancreatic cancer. Cochrane Database Syst Rev 2016;4. [PubMed]
- de Rooij T, van Hilst J, Vogel JA, et al. Dutch Pancreatic Cancer Group. Minimally invasive versus open distal pancreatectomy (LEOPARD): study protocol for a randomized controlled trial. Trials 2017;18:166. [Crossref] [PubMed]
- van Hilst J. Presentation at SAGES meeting Seattle April 13, 2018
- Kooby DA, Hawkins WG, Schmidt CM, et al. A Multicenter Analysis of Distal Pancreatectomy for Adenocarcinoma: Is Laparoscopic Resection Appropriate? J Am Coll Surg 2010;210:779-85. [Crossref] [PubMed]
- Shin SH, Kim SC, Song KB, et al. A Comparative Study of Laparoscopic vs. Open Distal Pancreatectomy for Left-Sided Ductal Adenocarcinoma: A Propensity Score-Matched Analysis. J Am Coll Surg 2015;220:177-85. [Crossref] [PubMed]
- Van Hilst J, de Rooij T, Abu Hilal M, et al. Worldwide survey on current use, value and safe implementation of minimally invasive pancreatic resection. HPB 2017;19:S40-108. [Crossref]
- Ejaz A, Sachs T, He J, et al. A comparison of open and minimally invasive surgery for hepatic and pancreatic resections using the Nationwide Inpatient Sample. Surgery 2014;156:538-47. [Crossref] [PubMed]
- Goh BK, Tan YM, Chung YF, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg 2008;143:956-65. [Crossref] [PubMed]
- Uranues S, Alimoglu O, Todoric B, et al. Laparoscopic resection of the pancreatic tail with splenic preservation. Am J Surg 2006;192:257-61. [Crossref] [PubMed]
- Justin V, Fingerhut A, Khatkov I, et al. Laparoscopic pancreatic resection-a review Transl Gastroenterol Hepatol 2016;1:36-43. [Crossref] [PubMed]
- Pryor A, Means JR, Pappas TN. Laparoscopic distal pancreatectomy with splenic preservation. Surg Endosc 2007;21:2326-30. [Crossref] [PubMed]
- Casadei R, Ricci C, D'Ambra M, et al. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: a case-control study. Updates Surg 2010;62:171-4. [Crossref] [PubMed]
- Briggs CD, Mann CD, Irving GR, et al. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg 2009;13:1129-37. [Crossref] [PubMed]
- Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg 1988;123:550-3. [Crossref] [PubMed]
- Sa Cunha A, Carrere N, Meunier B, et al. Stump closure reinforcement with absorbable fibrin collagen sealant sponge (TachoSil®) does not prevent pancreatic fistula after distal pancreatectomy: the FIABLE* multicenter controlled randomized study. Am J Surg 2015;210:739-48. [Crossref] [PubMed]
- Finan KR, Cannon EE, Kim EJ, et al. Laparoscopic and open distal pancreatectomy: a comparison of outcomes. Am Surg 2009;75:671-9. [PubMed]
- Tseng WH, Canter RJ, Bold RJ. Perioperative outcomes for open distal pancreatectomy: current benchmarks for comparison. J Gastrointest Surg 2011;15:2053-8. [Crossref] [PubMed]
- Borja-Cacho D, Al-Refaie WB, Vickers SM, et al. Laparoscopic distal pancreatectomy. J Am Coll Surg 2009;209:758-65. [Crossref] [PubMed]
- DiNorcia J, Schrope BA, Lee MK, et al. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. J Gastrointest Surg 2010;14:1804-12. [Crossref] [PubMed]
- Soh YF, Kow AW, Wong KY, et al. Perioperative outcomes of laparoscopic and open distal pancreatectomy: our institution's 5-year experience. Asian J Surg 2012;35:29-36. [Crossref] [PubMed]
- Nakamura Y, Uchida E, Aimoto T, et al. Clinical outcome of laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Surg 2009;16:35-41. [Crossref] [PubMed]
- Cho CS, Kooby DA, Schmidt CM, et al. Laparoscopic versus open left pancreatectomy: can preoperative factors indicate the safer technique? Ann Surg 2011;253:975-80. [Crossref] [PubMed]
- de Rooij T, Jilesen AP, Boerma D, et al. A nationwide comparison of laparoscopic and open distal pancreatectomy for benign and malignant disease. J Am Coll Surg 2015;220:263-70.e1. [Crossref] [PubMed]
- Khaled YS, Malde DJ, Packer J, et al. A case-matched comparative study of laparoscopic versus open distal pancreatectomy. Surg Laparosc Endosc Percutan Tech 2015;25:363-7. [Crossref] [PubMed]
- Diener MK, Seiler CM, Rossion I, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 2011;377:1514-22. [Crossref] [PubMed]
- Jensen EH, Portschy PR, Chowaniec J, et al. Meta-analysis of bioabsorbable staple line reinforcement and risk of fistula following pancreatic resection. J Gastrointest Surg 2013;17:267-72. [Crossref] [PubMed]
- Wang K, Fan Y. Minimally Invasive Distal Pancreatectomy: Review of the English Literature. J Laparoendosc Adv Surg Tech A 2017;27:134-40. [Crossref] [PubMed]
- Kleeff J, Diener MK, Z’graggen K, et al. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg 2007;245:573-82. [Crossref] [PubMed]
- Reeh M, Nentwich MF, Bogoevski D, et al. High surgical morbidity following distal pancreatectomy: still an unsolved problem. World J Surg 2011;35:1110-7. [Crossref] [PubMed]
- Bassi C, Butturini G, Falconi M, et al. Prospective randomized pilot study of management of the pancreatic stump following distal resection. HPB 1999;1:203-7. [Crossref]
- Knaebel HP, Diener MN, Wente MW, et al. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg 2005;92:539-46. [Crossref] [PubMed]
- Zhou W, Lv R, Wang X, et al. Stapler vs suture closure of pancreatic remnant after distal pancreatectomy: a meta-analysis. Am J Surg 2010;200:529-36. [Crossref] [PubMed]
- Ferrone CR, Warshaw AL, Rattner DW, et al. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg 2008;12:1691-7. [Crossref] [PubMed]
- Hamilton NA, Porembka MR, Johnston FM, et al. Mesh reinforcement of pancreatic transection decreases incidence of pancreatic occlusion failure for left pancreatectomy: a single-blinded, randomized controlled trial. Ann Surg 2012;255:1037-42. [Crossref] [PubMed]
- Kawai M, Hirono S, Okada KI, et al. Randomized controlled trial of pancreaticojejunostomy versus stapler closure of the pancreatic stump during distal pancreatectomy to reduce pancreatic fistula. Ann Surg 2016;264:180-7. [Crossref] [PubMed]
- Asbun HJ, Stauffer JA. Laparoscopic approach to distal and subtotal pancreatectomy: a clockwise technique. Surg Endosc 2011;25:2643-9. [Crossref] [PubMed]
- Ariyarathenam AV, Bunting D, Somaiah A. Laparoscopic Distal Pancreatectomy Using the Modified Prolonged Prefiring Compression Technique Reduces Pancreatic Fistula. J Laparoendosc Adv Surg Tech A 2015;25:821-5. [Crossref] [PubMed]
- Hirashita T, Ohta M, Yada K, et al. Effect of pre-firing compression on the prevention of pancreatic fistula in distal pancreatectomy. Am J Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg 1999;229:693-8. [Crossref] [PubMed]
- Baker MS, Bentrem DJ, Ujiki MB, et al. A prospective single institution comparison of peri-operative outcomes for laparoscopic and open distal pancreatectomy. Surgery 2009;146:635-43. [Crossref] [PubMed]
- Lee SY, Allen PJ, Sadot E, et al. Distal pancreatectomy: A single institution’s experience in open, laparoscopic, and robotic approaches. J Am Coll Surg 2015;220:18-27. [Crossref] [PubMed]
- Mabrut JY, Fernandez-Cruz L, Azagra JS, et al. Laparoscopic pancreatic resection: Results of a multicenter European study of 127 patients. Surgery 2005;137:597-605. [Crossref] [PubMed]
- Marangos IP, Buanes T, Rosok BI, et al. Laparoscopic resection of exocrine carcinoma in central and distal pancreas results in a high rate of radical resections and long postoperative survival. Surgery 2012;151:717-23. [Crossref] [PubMed]
- Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs open distal pancreatectomy a single-institution comparative study. Arch Surg 2010;145:616-21. [Crossref] [PubMed]
- Kooby DA, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg 2008;248:438-46. [PubMed]
- Jayaraman S, Gonen M, Brennan MF, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010;211:503-9. [Crossref] [PubMed]
- Rehman S, John SKP, Lochan R, et al. Oncological feasibility of laparoscopic distal pancreatectomy for adenocarcinoma: a single-institution comparative study. World J Surg 2014;38:476-83. [Crossref] [PubMed]
- Strasberg SM, Linehan DC, Hawkins WG. Radical Antegrade Modular Pancreatosplenectomy Procedure for Adenocarcinoma of the Body and Tail of the Pancreas: Ability to Obtain Negative Tangential Margins. J Am Coll Surg 2007;204:244-9. [Crossref] [PubMed]
- de Rooij T, Sitarz R, Busch OR, et al. Technical Aspects of Laparoscopic Distal Pancreatectomy for Benign and Malignant Disease: Review of the Literature. Gastroenterol Res Pract 2015;2015. [Crossref] [PubMed]
- Kang CM, Lee SH, Lee WJ. Minimally invasive radical pancreatectomy for left-sided pancreatic cancer: current status and future perspectives World J Gastroenterol 2014;20:2343-51. [Crossref] [PubMed]
- Hu M, Zhao G, Wang F, et al. Laparoscopic versus open distal splenopancreatectomy for the treatment of pancreatic body and tail cancer: a retrospective, mid-term follow-up study at a single academic tertiary care institution. Surg Endosc 2014;28:2584-91. [Crossref] [PubMed]
- Magge D, Gooding W, Choudry H, et al. Comparative effectiveness of minimally invasive open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg 2013;148:525-31. [Crossref] [PubMed]
- Limongelli P, Belli A, Russo G, et al. Laparoscopic and open surgical treatment of left-sided pancreatic lesions: clinical outcomes and cost-effectiveness analysis. Surg Endosc 2012;26:1830-6. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc 2010;24:1533-41. [Crossref] [PubMed]
- Stauffer JA, Rosales-Velderrain A, Goldberg RF, et al. Comparison of open with laparoscopic distal pancreatectomy: a single institution's transition over a 7-year period. HPB (Oxford) 2013;15:149-55. [Crossref] [PubMed]
- Tol JA, Gouma DJ, Bassi C, et al. International Study Group on Pancreatic Surgery. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014;156:591-600. [Crossref] [PubMed]
- Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-74. [Crossref] [PubMed]
- Sun W, Leong CN, Zhang Z, et al. Proposing the lymphatic target volume for elective radiation therapy for pancreatic cancer: a pooled analysis of clinical evidence. Radiat Oncol 2010;5:28. [Crossref] [PubMed]
- Sahakyan MA, Rosok BI, Kazaryan AM, et al. Impact of obesity on surgical outcomes of laparoscopic distal pancreatectomy: a Norwegian single-center study. Surgery 2016;160:1271-8. [Crossref] [PubMed]
- Sahakyan MA, Bjorn E, Kazaryan AM, et al. Perioperative outcomes and survival in elderly patients undergoing laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Sci 2017;24:42-8. [Crossref] [PubMed]
- Souche R, Fuks D, Perinel J, et al. Impact of laparoscopy in patients aged over 70 years requiring distal pancreatectomy: a French multicentric comparative study. Surg Endosc 2018;32:3164-73. [Crossref] [PubMed]
- Conlon KC, de Rooij T, van Hilst J, et al. Minimally Invasive Pancreatic Resection Organizing Committee. Minimally invasive pancreatic resections: cost and value perspectives. HPB (Oxford) 2017;19:225-33. [Crossref] [PubMed]
Cite this article as: Fingerhut A, Uranues S, Khatkov I, Boni L. Laparoscopic distal pancreatectomy: better than open? Transl Gastroenterol Hepatol 2018;3:49.