Role of secretory clusterin in hepatocarcinogenesis
Introduction
The death of patients with primary hepatic carcinoma (PHC) is one of the most common cancer-related deaths in the world (1). The multiple etiopathogenic factors involved include chronic hepatitis B or C virus (HBV or HCV) infection (2,3), nonalcoholic steatohepatitis, aflatoxin B1 intake (4,5), that induce the activation of oncogenes and the inhibition of oncosuppressor genes (6). PHC prognosis strongly depends on early diagnosis and effectiveness of treatment. However, although great progress of PHC therapy has been made, patients’ prognosis remains very poor because of postoperative relapse or metastases. Therefore, the knowledge of PHC markers, to evaluate clinical staging or monitor metastasis (7), is essential to improve the patients’ treatment. Recent studies have shown the usefulness of the clinical application of biomarkers such as the evaluation of the presence of circulating tumor cells, as well as of the expression of key signal molecules, long non-coding RNA, and microRNAs involved in PHC pathogenesis (8).
Clusterin (CLU) is considered a molecular chaperone that can be produced under the perturbation of many physical and chemical factors (9). CLU was first described in human liver cancer by Tobe et al. (10), that provided evidence that the gene (that they named CLI gene) encoding the glycoprotein SP-40,40 (also named apolipoprotein J, sulfated glycoprotein 2, SP-40, or testosterone-repressed prostate message 29) is located in the chromosome 8. Two CLU protein isoforms are present in human cells: a nuclear form (nCLU), proapoptotic, and a secretory form (sCLU) that is prosurvival (11). The predominant sCLU expression locates in the endoplasmic reticulum of certain tumor cells. Recently, circulating sCLU has been shown to be a valuable marker for diagnosis and surveillance of PHC (12,13). However, the exact relationship between abnormal sCLU expression and malignant transformation of hepatocytes is still largely unknown. This paper reviews the new advances of the knowledge of the relationships between sCLU and the malignant transformation of hepatocytes.
CLU structure and functions
The human CLU gene, constituted by 9 exons and 8 introns, is located on chromosome 8p21-p12. It encodes a 2,877 bp mRNA that is translated into a 449-amino acids polypeptide (14). Two CLU isoforms are known: the cytoplasmic sCLU (75~80 kDa) and the truncated nuclear nCLU (55 kDa). The sCLU, the major product of the CLU gene, is a highly conserved heterodimeric disulfide-linked polypeptide, widely present in human tissues or body fluids. It plays important roles in various physiological processes as well as in many pathological disturbance states. These include immune regulation, ageing, tissue remodeling, lipid transport, membrane recycling, complements cascade, DNA repair, cell adhesion, and cell-cell interactions, cancer progression, vascular damage, diabetes, kidney and neuron degeneration (15,16).
sCLU is also implicated in the, epithelial-mesenchymal transition (17), malignant transformation of hepatocytes and induction of metastasis. It interacts with oncogenes or suppressor genes, and related signal pathways, and is implicated in multiple drug resistance (MDR) (18,19). Hepatic sCLU is often adaptively overexpressed in hypoxic microenvironment and this contributes to increase tumorigenicity, metastatic potential, and MDR (20). Furthermore, sCLU could inhibit cell apoptosis induced by activated Bax, or protect liver cancer cells from apoptosis induced by endoplasmic reticulum stress, by interacting with the glucose-regulated apoptogenic protein 78 (21,22). sCLU may also interfere with the AKT signaling. Indeed, sCLU by forming a complex with EIF3I (eukaryotic translation initiation factor 3 subunit I) protein, prevents its degradation, thus contributing to the up-regulation of EIF3I/AKT/MMP13 signaling in hepatocellular carcinoma (HCC) (23).
Alteration of sCLU in hepatocarcinogenesis
Studies on hepatocarcinogenesis, induced in Wistar rats by chemical carcinogens, showed that differences in the expression of blood and liver sCLU could represent specific markers of liver cancer (24). Indeed, circulating and liver sCLU concentrations gradually increase during hepatocarcinogenesis. Furthermore, immunohistochemical determination showed sCLU positivity in hepatocytes four weeks after initiation, that gradually increase around the portal area, at the 8th week, and reached its maximum in liver parenchyma at the 21st week. These findings suggest that sCLU plays a role during liver carcinogenesis (25,26).
As yet, the pathogenesis of PHC has not been fully elucidated. Chronic inflammation and persistent HBV or HCV infection should be implicated in the transformation of liver stem cells (LSC) to cancer stem cells (CSC). Although the pathogenetic role of hepatic sCLU activation in PHC has not yet been fully elucidated, the abnormal sCLU expression could be useful for early diagnosis or could be considered for targeted therapy (27). Also, CLU plays a key role in maintaining the integrity of endoplasmic reticulum during drug-induced stress and drug resistance mechanism of CSC. Hence, down-regulation or suppression of CLU gene transcription could significantly alter MDR of liver cancer cells (28).
Tissues sCLU in human PHC
Great efforts have been made in the past decade to explore the mechanisms of PHC invasiveness and metastasis. The alterations of liver sCLU expression at mRNA or protein level were investigated in PHC and non-tumor surrounding tissue (13). No significant difference of the sCLU mRNA level was found between patients with stage I PHC and the non-tumor controls, but drastic sCLU up-regulation occurred in patients with PHC from staging II to IV. Immunohistochemistry showed sCLU positivity in the cytoplasm of both PHC and non-tumor tissue. However, the positivity in the PHC group, amounting to 73.3% at stage I, 37.5% at stage II, 68% at stage III, and 88.9% at stage IV, was significantly higher than that in the non-tumor group (23.3%) (29,30). Growing evidence indicates that sCLU plays an important role, as a molecular chaperone, in PHC cell proliferation and metastasis formation. The level of circulating sCLU mRNA and protein gradually increased with the PHC clinical stage. These findings strongly suggest that sCLU expression could be a valuable biomarker to distinguish malignant from benign liver nodular lesions (31,32).
Circulating sCLU expression
The evaluation of the presence of sCLU in PHC patents’ serum has shown that high level of circulating sCLU is associated with poor prognosis (33,34). These studies revealed an average serum sCLU level significantly higher in the PHC group than in liver cirrhosis, chronic hepatitis, and normal controls. The receiver operating characteristic (ROC) curve showed that a serum CLU value of 50 µg/mL produced the best sensitivity (91%) and specificity (83%) for differentiating HCC patients with HBV-related cirrhosis from those with HBV-related cirrhosis alone (33). A comparison with alpha-fetoprotein (AFP), as a PHC marker, showed that serum CLU is more sensitive and specific than serum AFP for differentiating HCC from cirrhosis patients (33). The clinicopathological features of PHC patients with higher sCLU levels in sera indicate a high increase in the serum sCLU in patients with poorly differentiated tumors, portal vein invasion and lymph node infiltration (34). The sCLU is associated with greater ROC area under the curve (0.95) than AFP (0.85) (34). If the cutoff value is 128 µg/mL as a limit, serum sCLU level represents a useful marker for PHC diagnosis with high sensitivity (90%) and specificity (87%) (34). This indicates that the abnormal sCLU level is a useful molecular marker for PHC diagnosis (12,13). Analogous conclusions were reached when sCLU linked to Datura stramonium lectin (DSL; DSL reactive sCLU) was considered (35), indicating that the circulating glycosylated sCLU levels represent a more sensitive PHC marker than AFP.
Conclusions
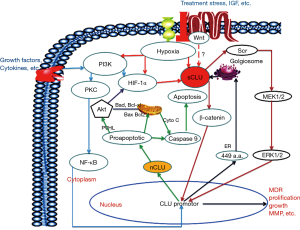
In conclusion, the up-regulation of sCLU expression is considered to promote PHC incidence, this could be related to the upregulation of different signaling pathways (Figure 1). The circulating sCLU level may be considered a novel biomarker of PHC, useful for the diagnosis and the individualized treatment of the disease. In addition, sCLU could improve MDR of PHC patients (36,37) and could contribute to HCC cell migration, EMT and formation of metastases. Therefore, the inhibition of sCLU expression, by specific CLU inhibitors, could represent a new targeted therapy that could improve the effects of PHC chemotherapy (38,39).
Acknowledgements
Funding: This work was supported by National Natural Science Foundation (No. 81673241 and 81702419); Projects of Jiangsu Medical Science (No. BE2016698) and Graduate innovation (No. KYCX17_1934); Nantong Health and Family Planning Commission (No. WQ2016083); and National Int S. & T. Cooperation Program (No. 2013DFA32150) of China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen JG, Zhu J, Zhang YH, et al. Cancer survival in Qidong between 1972 and 2011: a population-based analysis. Mol Clin Oncol 2017;6:944-54. [Crossref] [PubMed]
- Tong Hv, Bock CT, Velavan TP. Genetic insights on host and hepatitis B virus in liver diseases. Mutat Res Rev Mutat Res 2014;762:65-75. [Crossref] [PubMed]
- Matsuura K, Tanaka Y. Host genetic variations associated with disease progression in chronic hepatitis C virus infection. Hepatol Res 2018;48:127-33. [Crossref] [PubMed]
- Hernández-Aquino E, Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J Gastroenterol 2018;24:1679-707. [Crossref] [PubMed]
- Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin 2012;62:394-9. [Crossref] [PubMed]
- Machairas N, Papaconstantinou D, Stamopoulos P, et al. The emerging role of laparoscopic liver resection in the treatment of recurrent hepatocellular carcinoma: a systematic review. Anticancer Res 2018;38:3181-6. [PubMed]
- Choi YM, Lee SY, Kim BJ. Naturally occurring hepatitis B virus reverse transcriptase mutations related to potential antiviral drug resistance and liver disease progression. World J Gastroenterol 2018;24:1708-24. [Crossref] [PubMed]
- Xie M, Yang Z, Liu Y, et al. The role of HBV-induced autophagy in HBV replication and HBV related-HCC. Life Sci 2018;205:107-12. [Crossref] [PubMed]
- Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res 2014;24:92-104. [Crossref] [PubMed]
- Tobe T, Minoshima S, Yamase S, et al. Assignment of a human serum glycoprotein SP-40,40 gene (CLI) to chromosome 8. Cytogenet Cell Genet 1991;57:193-5. [Crossref] [PubMed]
- Zhong J, Yu X, Dong X, et al. Therapeutic role of meloxicam targeting secretory clusterin-mediated invasion in hepatocellular carcinoma cells. Oncol Lett 2018;15:7191-9. [PubMed]
- Zheng WJ, Sai WL, Yao M, et al. Down-regulated clusterin expression enhances sensitivity of hepatoma cells to anti-cancer drugs. Zhonghua Gan Zang Bing Za Zhi 2015;23:844-8. [PubMed]
- Zheng W, Yao M, Sai W, et al. Diagnostic and prognostic significance of secretory clusterin expression in patients with hepatocellular carcinoma. Tumour Biol 2016;37:999-1008. [Crossref] [PubMed]
- Wong P, Pineault J, Lakins J, et al. Genomic organization and expression of the rat TRPM-2 (clusterin) gene, a gene implicated in apoptosis. J Biol Chem 1993;268:5021-31. [PubMed]
- Lourda M, Trougakos IP, Gonos ES. Development of resistance to chemo- therapeutic drugs in human osteosarcoma cell lines largely depends on up- regulation of Clusterin/Apolipoprotein J. Int J Cancer 2007;120:611-22. [Crossref] [PubMed]
- Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic Res 2006;40:1324-34. [Crossref] [PubMed]
- Wang C, Jiang K, Kang X, et al. Tumor-derived secretory clusterin induces epithelial-mesenchymal transition and facilitates hepatocellular carcinoma metastasis. Int J Biochem Cell Biol 2012;44:2308-20. [Crossref] [PubMed]
- Zhong J, Yu X, Dong X, et al. Downregulation of secreted clusterin potentiates the lethality of sorafenib in hepatocellular carcinoma in association with the inhibition of ERK1/2 signals. Int J Mol Med 2018;41:2893-900. [PubMed]
- Wang X, Zou F, Zhong J, et al. Secretory clusterin mediates oxaliplatin resistance via the Gadd45a/PI3K/Akt signaling pathway in hepatocellular carcinoma. J Cancer 2018;9:1403-13. [Crossref] [PubMed]
- Xiu P, Dong X, Dong X, et al. Secretory clusterin contributes to oxaliplatin resistance by activating Akt pathway in hepatocellular carcinoma. Cancer Sci 2013;104:375-82. [Crossref] [PubMed]
- Xiu P, Xu Z, Liu F, et al. Downregulating sCLU enhances the sensitivity of hepatocellular carcinoma cells to gemcitabine by activating the intrinsic apoptosis pathway. Dig Dis Sci 2014;59:1798-809. [Crossref] [PubMed]
- Petrillo M, Patella F, Pesapane F, et al. Hypoxia and tumor angiogenesis in the era of hepatocellular carcinoma transarterial loco-regional treatments. Future Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Wang C, Jin G, Jin H, et al. Clusterin facilitates metastasis by EIF3I/Akt/ MMP13 signaling in hepatocellular carcinoma. Oncotarget 2015;6:2903-16. [PubMed]
- Sheng X, Huang T, Qin J, et al. Identification of the differential expression profiles of serum and tissue proteins during rat hepatocarcinogenesis. Technol Cancer Res Treat 2018;17. [Crossref] [PubMed]
- Zheng W, Yao M, Qian Q, et al. Oncogenic secretory clusterin in hepatocellular carcinoma: expression at early staging and emerging molecular target. Oncotarget 2016;8:52321-32. [PubMed]
- Xiu P, Dong XF, Li XP, et al. Clusterin: review of research progress and looking ahead to direction in hepatocellular carcinoma. World J Gastroenterol 2015;21:8262-70. [Crossref] [PubMed]
- Mitra A, Yan J, Xia X, et al. IL6-mediated inflammatory loop reprograms normal to epithelial-mesenchymal transition+ metastatic cancer stem cells in preneo-plastic liver of transforming growth factor beta-deficient β2-spectrin+/- mice. Hepatology 2017;65:1222-36. [Crossref] [PubMed]
- Arumugam P, Samson A, Ki J, et al. Knockdown of clusterin alters mitochondrial dynamics, facilitates necrosis in camptothecin-induced cancer stem cells. Cell Biol Toxicol 2017;33:307-21. [Crossref] [PubMed]
- Lau SH, Sham JS, Xie D, et al. Clusterin plays an important role in hepato- cellular carcinoma metastasis. Oncogene 2006;25:1242-50. [Crossref] [PubMed]
- Kang YK, Hong SW, Lee H, et al. Overexpression of clusterin in human hepatocellular carcinoma. Hum Pathol 2004;35:1340-6. [Crossref] [PubMed]
- Chen RX, Song HY, Dong YY, et al. Dynamic expression patterns of differential proteins during early invasion of hepatocellular carcinoma. PLoS One 2014;9. [Crossref] [PubMed]
- Lai JP, Chen ZM, Lok T, et al. Immunohistochemical stains of proliferating cell nuclear antigen, insulin-like growth factor 2 and clusterin help distinguish malignant from benign liver nodular lesions. J Clin Pathol 2014;67:464-9. [Crossref] [PubMed]
- Wang Y, Liu YH, Mai SJ, et al. Evaluation of serum clusterin as a surveillance tool for human hepatocellular carcinoma with hepatitis B virus related cirrhosis. J Gastroenterol Hepatol 2010;25:1123-8. [Crossref] [PubMed]
- Nafee AM, Pasha HF, Abd El Aal SM, et al. Clinical significance of serum clusterin as a biomarker for evaluating diagnosis and metastasis potential of viral-related hepatocellular carcinoma. Clin Biochem 2012;45:1070-4. [Crossref] [PubMed]
- Comunale MA, Wang M, Rodemich-Betesh L, et al. Novel changes in glycosylation of serum Apo-J in patients with hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 2011;20:1222-9. [Crossref] [PubMed]
- Zheng W, Sai W, Yao M, et al. Silencing clusterin gene transcription on effects of multidrug resistance reversing of human hepatoma HepG2/ADM cells. Tumour Biol 2015;36:3995-4003. [Crossref] [PubMed]
- Wang C, Jiang K, Gao D, et al. Clusterin protects hepatocellular carcinoma cells from endoplasmic reticulum stress induced apoptosis through GRP78. PLoS One 2013;8. [Crossref] [PubMed]
- Mukaida N, Nakamoto Y. Emergence of immunotherapy as a novel way to treat hepatocellular carcinoma. World J Gastroenterol 2018;24:1839-58. [Crossref] [PubMed]
- Heinrich B, Czauderna C, Marquardt JU. Immunotherapy of hepatocellular carcinoma. Oncol Res Treat 2018;41:292-7. [Crossref] [PubMed]
Cite this article as: Yao M, Fang M, Zheng W, Dong Z, Yao D. Role of secretory clusterin in hepatocarcinogenesis. Transl Gastroenterol Hepatol 2018;3:48.


