Utility and performance characteristics of a novel submucosal injection agent (EleviewTM) for endoscopic mucosal resection and endoscopic submucosal dissection
Introduction
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are being increasingly adopted worldwide for management of early esophageal and gastric cancers, as well as large colon polyps (1,2). These techniques are being favored over invasive surgery given their favorable outcomes. There is ongoing research targeted at making these endoscopic procedures simplified and time efficient, which includes development of new tools for dissection, hemostasis and submucosal elevation prior to resection (1,2).
An important aspect of successful endoscopic resection is a sufficient and long-lasting submucosal cushion formation, wherein fluid is injected between the lesion and deeper submucosal layers, to allow safe and en bloc removal of the target lesions, and preventing adverse events, like bleeding and perforation. The most commonly used solution to achieve the submucosal cushion is normal saline (NS), but it carries a major limitation of quick absorption into the adjacent mucosa, thus necessitating frequent injections to recreate cushion and maintain mucosal elevation. To overcome this limitation, endoscopists have used several other viscous solutions, namely 50% dextrose (3,4), glycerol, sodium hyaluronic acid (5), succinylated gelatin (6), hydroxyethyl starch (7), fibrinogen mixture (8), and mesna (9), with varying degrees of success. Two recent meta-analyses have clearly demonstrated superiority of other viscous solutions over NS for EMR and ESD (10,11), but research to determine the ideal submucosal injection is still ongoing.
EleviewTM (Cosmo pharmaceuticals NV, Dublin, Ireland) is a sterile, clear emulsion, packaged as 10 mL ampoules, which is pre-stained with methylene blue and can be injected using standard available injection needles. The contents of this emulsion include water, medium chain triglycerides, sodium chloride, polyoxyl-15-hydroxystearate, methylene blue and poloxamer-188. EleviewTM was approved in the USA by the Food and Drug Administration (FDA) in September 2015 and in the European Union in June 2016, for use as a submucosal injection agent during EMR, ESD and polypectomy procedures in the gastrointestinal tract (12). It is designed to provide a submucosal cushion of optimal height and duration, allowing the endoscopist an easier and safer resection procedure. We evaluated the utility and performance characteristics of this novel submucosal injection agent (EleviewTM) for EMR and ESD.
Methods
This study was performed at the Stanford University School of Medicine, which is a regional referral center for management of large gastrointestinal polyps and early cancers. This is a single center series, where one experienced endoscopist (Shai Friedland) performed all the EMR/ESD procedures. The study was approved by Stanford institutional review board (IRB) (#20122).
Inclusion criteria
Consecutive adult patients referred for EMR or ESD during February–March 2017 were considered. All patients signed the pertinent consent forms for resection.
Exclusion criteria
Patients who were high-risk for endoscopic intervention (EMR/ESD), were excluded. Additionally, patients with known allergies to components of EleviewTM were excluded as well.
EleviewTM
This FDA approved submucosal injection agent was made commercially available in the USA in March 2017. It was obtained through Cosmo Pharmaceuticals NV.
Data collected
Demographics (age, gender and race) and indications for referral were noted for every patient. Endoscopic data collected included type, location and size of polyp, type of intervention performed (EMR or ESD), resection technique (en bloc or piece-meal), details of submucosal injection agent (volume injected, number of injections, duration of lift with each injection), amount of time required for complete resection, pathology of resected specimen, achievement of R0 margin, and any complications (bleeding, perforation and post-polypectomy syndrome/abdominal pain). All data was collected in a HIPAA protective manner and handled only by the authors. The data was stored on password-protected computer, which was stored behind lock-and-key.
Results
Ten consecutive patients referred for large colon polyp EMR/ESD, and one patient each for gastric and esophageal lesion EMR/ESD, were enrolled. The demographic information, type of procedure performed, and overall complication profile of enrolled patients is provided in Table 1.
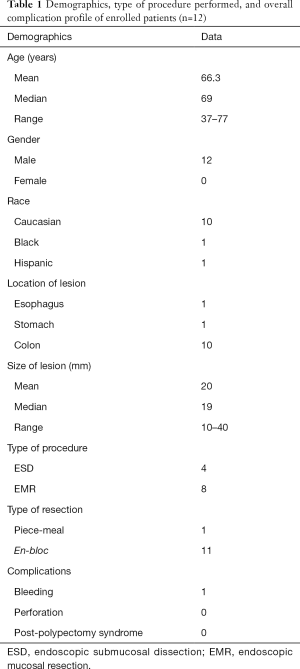
Full table
All twelve patients underwent successful resection procedures, 4 ESD and 8 EMR, with lesion size ranging between 10–40 mm, out of which 11 were completed as en-bloc resections. EleviewTM was used as the submucosal lifting agent for all these procedures, and details of its performance characteristics are listed in Table 2.

Full table
The amount of EleviewTM injected ranged from 3–10 cc, using 1–5 injections during these resection procedures. For all EMR procedures, the lift duration achieved with single injection of EleviewTM (3–5 cc) lasted longer than the duration of EMR procedure (2–3 minutes) and additional injection was not required in any patient. On the other hand, during the four ESD procedures, EleviewTM had to be injected between 3–5 times (total 10 cc), and the average lift duration was noted to be 12.5 minutes (range, 10–15 minutes), while the ESD procedures lasted 60–140 minutes. After injection of a total of 10 cc of Eleview during ESD, subsequent injections were performed with saline (with methylene blue and epinephrine).
R0 resection was achieved in 10/12 patients. Pathology on the resected specimens revealed cancer in 1 patient, high-grade dysplasia (HGD) in 1 patient, low-grade dysplasia (LGD) in 8 patients, and benign in 2 patients (hyperplastic, and muco-submucosal elongated polyp). Only one patient had minor bleeding, which was managed by snare tip coagulation followed by clipping. No post-polypectomy pain syndrome or perforation was noted in this series.
The endoscopist graded the ease of performance of endoscopic resection, and all EMRs were rated easy, and notably the submucosal lift lasted longer in these than the duration of resection (Figure 1). For ESDs, only one out of four was rated easy, and remaining three were rated moderately-very difficult, where multiple injections were needed to maintain the submucosal lift to accomplish a complete resection (Figure 2).
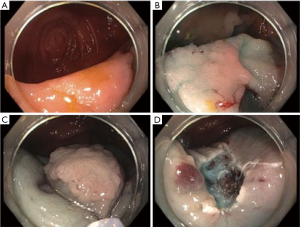
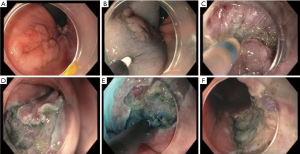
Discussion
This is the first report of utility and performance characteristics of a recently FDA-approved and now commercially available submucosal injection agent, EleviewTM, which includes EMR and ESD patients. EleviewTM is a patented composition (SIC 8000), which comes pre-stained with methylene blue, and injected between the mucosal layers where it clearly separates the mucosal layers for more than 60 minutes in preclinical testing, thus potentially allowing the endoscopist sufficient time to resect polyps/tumors safely without perforation and bleeding (12).
Submucosal elevation with fluid injection is a critical step of EMR and ESD, and has traditionally been achieved using mixture of NS with epinephrine. The purpose of this elevation is to create a fluid cushion between the lesion and the deeper layers, allowing complete en bloc resection, while minimizing conduction of current to deeper layers to minimize complications, such as perforation, bleeding and post-polypectomy syndrome. A major limitation of NS is quick tissue absorption into adjacent mucosa resulting in flattening of the submucosal elevation, and requiring repeated injections. Alternative submucosal agents are available, each with their merits and limitations, including sodium hyaluronate (viscoelastic solution with longer duration submucosal elevation; expensive), succinylated gelatin (clear, inexpensive colloid) and 50% dextrose (easily available and inexpensive hypertonic solution, but not as long lasting) (3-6). Many of the available submucosal injection solutions can maintain submucosal elevation for longer time than NS, but there is still no one universally accepted one as a standard of care. The quintessentially ideal submucosal injection agent is the one that is easily available at a low cost, provides adequate height and long duration of submucosal elevation without tissue absorption, while being safe to the injected tissues and not interfering with the histologic interpretation of the resected specimen.
Our preliminary results suggest that EleviewTM is a robust submucosal injection agent for the performance of a safe and efficient EMR. The lift, however, lasts for average of 12.5 minutes (range, 10–15 minutes), which is mostly inadequate for completion of an ESD. In our series, the four patients undergoing ESD required repeated injections (3–5 times) to sustain submucosal elevation for the duration of the procedure. Hence our experience is in conflict with the reported submucosal elevation duration of ~60 minutes that has been observed in earlier pig models. In our human EMR/ESD, the mean duration of lift was much less. Nevertheless, EleviewTM allowed safe and complete (10/12 R0) resection in 100% patients (n=12), with performance of resection graded by experienced endoscopist as easy for 100% of EMRs and 25% of ESDs.
The exact mechanism of action of EleviewTM on tissue specimens and the healing process are not entirely clear, as is the case with the other viscous solutions. The main content of EleviewTM emulsion includes poloxamer-188, a temperature-dependent liquid-to-gel transition material, which after injection reconfigures to occupy the submucosal space and forms a colored submucosal cushion of optimal height that pushes the mucosa away from the submucosal layer, allowing for an easier resection. Similar reverse phase poloxamers have been tested in the past in ex vivo and in vivo models, and have achieved more durable submucosal cushion for safe and efficient EMR (13). Recently, these poloxamers have been injected using high-pressure injection system, for more reliable submucosal cushion formation (14). Furthermore, EleviewTM appeared to be an effective alternative to saline solution for submucosal injection for EMR/ESD in porcine model (15), but data of its efficacy for ESD in humans is not available, except for our study.
The strengths of this study include its execution by a single experienced endoscopist and the consistency of type of snare and cautery used, which can favorably affect outcomes such as en bloc resection rates, achievement of R0 margins, and adverse events. This ensures credible historical comparison with other submucosal agents used in the performance of EMR/ESD. A conference abstract by Rex et al. on the multi-center experience with EleviewTM for ≥2 cm polyps, followed by a recent publication, supports our findings of safety of EleviewTM as a submucosal injection agent, but no ESDs were included in that study (16,17). Weaknesses of our study include the small number of patients studied that does not allow for firm conclusions to be made regarding its safety. For example, it is unclear if perforation or bleeding rates are positively or negatively affected by EleviewTM. We can nevertheless claim that in our preliminary experience, there was no negative impact on the histopathologic assessment or impaired hemostasis. Further, we cannot opine on the cost-effectiveness of the use of this agent as compared to NS or other agents used in mucosal lifting, but at a cost of $81 per ampule this remains a significant consideration and concern.
In summary, our study establishes some preliminary safety and utility performance characteristics of the first FDA-approved submucosal injection agent, EleviewTM. The average duration of lift was noted to be 12.5 minutes, which makes it a robust submucosal elevation agent for most EMRs which are expected to last under 10 minutes. We feel that repeated injections for maintenance of elevation will be required in longer ESD cases. Endoscopists contemplating transitioning from NS to EleviewTM as their preferred submucosal injection agent for EMR may need closer introspection of its marginal advantages over NS, while balancing its cost-effectiveness and understanding its limitations for longer resections including ESD. Larger, multi-center, prospective controlled trials are required to compare performance of EleviewTM to other available viscous submucosal solutions for ESD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Stanford institutional review board (IRB) (#20122). All patients signed the pertinent consent forms for resection.
References
- ASGE Technology Committee, Hwang JH, Konda V, et al. Endoscopic mucosal resection. Gastrointest Endosc 2015;82:215-26. [Crossref] [PubMed]
- ASGE Technology Committee, Maple JT, Abu Dayyeh BK, et al. Endoscopic submucosal dissection. Gastrointest Endosc 2015;81:1311-25. [Crossref] [PubMed]
- Hurlstone DP, Fu KI, Brown SR, et al. EMR using dextrose solution versus sodium hyaluronate for colorectal Paris type I and 0-II lesions: a randomized endoscopist-blinded study. Endoscopy 2008;40:110-4. [Crossref] [PubMed]
- Katsinelos P, Kountouras J, Paroutoglou G, et al. A comparative study of 50% dextrose and normal saline solution on their ability to create submucosal fluid cushions for endoscopic resection of sessile rectosigmoid polyps. Gastrointest Endosc 2008;68:692-8. [Crossref] [PubMed]
- Yamamoto H, Yahagi N, Oyama T, et al. Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid "cushion" in endoscopic resection for gastric neoplasms: a prospective multicenter trial. Gastrointest Endosc 2008;67:830-9. [Crossref] [PubMed]
- Moss A, Bourke MJ, Metz AJ. A randomized, double-blind trial of succinylated gelatin submucosal injection for endoscopic resection of large sessile polyps of the colon. Am J Gastroenterol 2010;105:2375-82. [Crossref] [PubMed]
- Fasoulas K, Lazaraki G, Chatzimavroudis G, et al. Endoscopic mucosal resection of giant laterally spreading tumors with submucosal injection of hydroxyethyl starch: comparative study with normal saline solution. Surg Laparosc Endosc Percutan Tech 2012;22:272-8. [Crossref] [PubMed]
- Lee SH, Park JH, Park DH, et al. Clinical efficacy of EMR with submucosal injection of a fibrinogen mixture: a prospective randomized trial. Gastrointest Endosc 2006;64:691-6. [Crossref] [PubMed]
- Sumiyama K, Toyoizumi H, Ohya TR, et al. A double-blind, block-randomized, placebo-controlled trial to identify the chemical assistance effect of mesna submucosal injection for gastric endoscopic submucosal dissection. Gastrointest Endosc 2014;79:756-64. [Crossref] [PubMed]
- Huai ZY, Feng Xian W, Chang Jiang L, et al. Submucosal injection solution for endoscopic resection in gastrointestinal tract: a traditional and network meta-analysis. Gastroenterol Res Pract 2015;2015. [Crossref] [PubMed]
- Yandrapu H, Desai M, Siddique S, et al. Normal saline solution versus other viscous solutions for submucosal injection during endoscopic mucosal resection: a systematic review and meta-analysis. Gastrointest Endosc 2017;85:693-9. [Crossref] [PubMed]
- Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K150852
- Fernández-Esparrach G, Shaikh SN, Cohen A, et al. Efficacy of a reverse-phase polymer as a submucosal injection solution for EMR: a comparative study (with video). Gastrointest Endosc 2009;69:1135-9. [Crossref] [PubMed]
- Pioche M, Ciocirlan M, Lépilliez V, et al. High-pressure jet injection of viscous solutions for endoscopic submucosal dissection: a study on ex vivo pig stomachs. Surg Endosc 2014;28:1742-7. [Crossref] [PubMed]
- Spadaccini M, Hassan C, Maselli R, et al. Efficacy and safety of SIC-8000 (Eleview®) for submucosal injection for endoscopic mucosal resection and endoscopic submucosal dissection in an in vivo porcine model. Dig Liver Dis 2018 ar;50:260-6.
- Rex D, Wallace MB, Sharma P, et al. Randomized double-blinded trial comparing effectiveness and safety of submucosal injection with SIC-8000 vs NS. Digestive Diseases Week 2017, Chicago, IL.
- Repici A, Wallace M, Sharma P, et al. A novel submucosal injection solution for endoscopic resection of large colorectal lesions: a randomized, double-blind trial. Gastrointest Endosc 2018. [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Girotra M, Triadafilopoulos G, Friedland S. Utility and performance characteristics of a novel submucosal injection agent (EleviewTM) for endoscopic mucosal resection and endoscopic submucosal dissection. Transl Gastroenterol Hepatol 2018;3:32.


