Does “July effect” exist in colonoscopies performed at teaching hospitals?
Introduction
Colonoscopy is one of the most consistently performed procedures to assess the wide variety of gastrointestinal conditions, as well as one of the primary screening and surveillance modalities to detect colorectal neoplasia (1). Although typically considered as a safe technique, colonoscopy can lead to a few serious and sometimes fatal adverse events such as colonic perforation, bleeding, infection and rarely splenic rupture (1,2). The incidence of these adverse events following colonoscopy is fairly low; colonic perforation (0.003% to 0.3%) (3-7), bleeding (0.1% to 0.6%) (3), infection (bacteremia) (0% to 25%) (8) and splenic rupture (0.001%) (9). The rate of hospitalization and median length of stay (LOS) due to the colonoscopy-related complications were found to be as low as 0.05% (95% CI: 0.00% to 0.26%) (10).
“July effect” is delineated as the influence of beginning of the academic year on the quality of the healthcare and patient care services due to the switchover of trained residents or fellows with inexperienced ones (11-13). Outcomes can vary between the studies while assessing the July effect, however, many large studies have shown a comparatively higher mortality due to various illnesses at the beginning of the academic year as compared to the later part of the academic year (13-17). Moreover, many studies have reported a negative impact on the quality of the patient care during the early academic months in the teaching hospitals by showing evidence of the prolonged hospital LOS and higher total hospital costs (18-23).
Typically, a gastroenterology fellow at the start of training performs the colonoscopy procedure under the direct supervision of an attending physician. Despite the fact that it is a safe procedure to perform, the likelihood of colonoscopy-related complications may be higher during this learning curve. We presume that the incidence of post-colonoscopy complications might be lower at the end of the academic year since by this time fellows have enough experience and skills to perform the colonoscopy. Our primary aim was to assess the existence of the “July effect” in the colonoscopies performed at urban-teaching hospitals by using the largest National Inpatient Sample (NIS) database. Therefore, we proposed to measure and compare the all-cause mortality and the incidence of post-colonoscopy complications along with the LOS and total hospitalization charges and disposition between the first 3 months (July to September) and the last 3 months (April to June) of the academic year.
Methods
Data source
Our study population was derived from the 2010–2014 Healthcare Cost and Utilization Project’s National Inpatient Sample (HCUP-NIS) database, which is funded by the Agency for Healthcare Research and Quality. The NIS is the largest publicly available all-payer inpatient healthcare database in the United States (US). This dataset is deliberated as a stratified sample from 20% nonfederal US community acute care hospitals, which represents 95% of the US population. It comprises of the more than 7 million unweighted discharges per year. Each hospitalization can be transformed into weighted (weight is calculated by the sum of discharges from all acute care hospitals in the US divided by the sum of discharges incorporated in the 20% sample) by discharge weight provided in the dataset to yield national estimates. The weighted dataset is intended to comprehend more than 35 million hospitalizations per year. Patients’ demographics, diagnoses, resource utilization including the LOS, procedures, and total hospitalization charges are integrated into the NIS. The hospitalization characteristics are classified in the manner of ownership/control, bed size, teaching status, urban/rural location, and geographic region. Both patient and hospital level data is incorporated in this dataset. The International Classification of Diseases, 9th revision, Clinical Modification (ICD-9 CM) coding system is used to collect up to 25 discharge diagnoses and 15 procedures on each hospitalization. Institutional Review Board (IRB) authorization was not mandatory for this de-identified dataset (24).
Study population
ICD-9 CM procedure codes 45.22, 45.23, 45.25, 45.42, and 45.43 were used to identify the adult patients (>18 years of age) who underwent inpatient colonoscopy. Patients were excluded if the information about the colonoscopy was missing or it was carried out before or on the day of admission, and/or if they were hospitalized to the non-teaching urban or rural hospitals. Post-colonoscopy complications were identified by using the ICD-9 CM codes which were applied for a secondary diagnosis to recognize the post-colonoscopy complications in patients who received a colonoscopy as outlined above. All the ICD-9 CM codes utilized were documented and validated in the previously published studies (25,26). Colonoscopies completed during the months of July, August and September were compared to those carried out during the months of April, May, and June in the urban-teaching hospitals.
Study variables
The analysis included baseline demographics of study cohort such as the age, sex, admission day, type of admission, race, median household income national quartile for patient zip code (first quartile: 0–25th; second quartile: 26–50th; third quartile: 51–75th; fourth quartile: 76–100th) and primary expected payer. The hospitalization characteristics such as the region of the hospital, bed size of hospital and control/ownership of the hospital, were also taken into consideration while performing the analyses.
Outcomes
The primary outcomes of this study were the all-cause mortality and the rate of post-colonoscopy complications including colonic perforation, post-colonoscopy hemorrhage/bleeding, postoperative infections, and splenic rupture. Secondary outcomes were the LOS, and total hospitalization charges and disposition. We also assessed the odds of complications during July–September vs. April–June after adjusting for potential confounding variables including age, sex, race, median household income national quartile for patient zip code and payer status.
Statistical analysis
Pearson’s Chi-square test and Student t-test were used for evaluating the categorical and continuous variables, respectively. The categorical and continuous variables were stated in percentages and mean ± SD, correspondingly. A two-tailed P value <0.05 was considered as the threshold of the statistical significance. Multivariate regression analysis was executed to evaluate the odds of complications during July–September vs. April–June after adjusting for potential confounding variables including age, sex, race, median household income national quartile for patient zip code and payer status. Multivariate logistic regression results were described by adjusted OR, 95% CI, and P value. SPSS version 22 (IBM Corp, Armonk, NY, USA) was utilized to perform all statistical analyses. The patients with Missing data of cohort were excluded in the statistical analysis. We built in statistical analysis with weighted data to produce nationwide estimates.
Results
Baseline demographics and hospital characteristics
In this cohort, we incorporated a total of 124,155 (weighted n=617,907) colonoscopy procedures which were performed in the urban-teaching hospitals of the US from January 2010 through December 2014. Out of these, 61,272 (weighted n=304,946) and 62,883 (weighted n=312,961) procedures were performed during early (July to September) and later (April to June) academic months, respectively. Demographics and hospitals variables were comparable in the patients undergoing the colonoscopy procedures during the early academic months (July to September) and later academic months (April to June). Baseline demographic and hospitals characteristics are shown in Table 1.
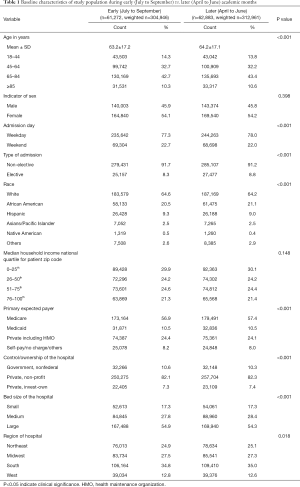
Full table
The all-cause mortality and post-colonoscopy complications
There was no significant difference in the all-cause mortality (1.4% vs. 1.4%, P=0.208), and the Post-colonoscopy complications such as colonic perforations (3.1% vs. 3.2%, P=0.229) and postoperative infections (0.6% vs. 0.6%, P=0.733) were also comparable between the two groups. Similarly, the incidence of splenic rupture (0.0% vs. 0.0%, P=0.180) was equally rare in both the groups. The incidence of bleeding/hematoma following colonoscopy (0.9% vs. 0.8%, P=0.004) was marginally higher during the later academic months (April to June) than the early academic months (July to September) (Table 2).
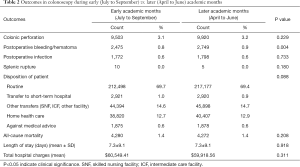
Full table
LOS and total hospitalization charges and disposition of patients post-colonoscopy
There were no distinctions in terms of LOS (days) (7.3±9.1 vs. 7.3±9.1, P=0.918) and total hospitalization charges ($60,549.41 vs. $59,918.56, P=0.311) following the colonoscopies between the early (July to September) and later (April to June) academic months. There was no statistical difference in the disposition of the patients to routine (69.7% vs. 69.4%), transfer to short-term hospital (1.0% vs. 0.9%), other transfers (skilled nursing facility, intermediate care facility, and other facility) (14.6% vs. 14.7%), home health care (12.7% vs. 12.9%) and against medical advice (0.6% vs. 0.6%) (P=0.088) following the colonoscopy procedures between the two groups (Table 2).
Odds of post-colonoscopy complications according to academic months
After regulating the plausible confounders, colonoscopy during the July–September months was not found to be a significant independent predictor for post-colonoscopy complications such as colon perforation (OR =0.99, 95% CI: 0.93–1.06, P=0.760), postoperative bleeding/hematoma (OR =0.92, 95% CI: 0.81–1.04, P=0.196) and postoperative infection (OR =0.99, 95% CI: 0.84–1.15, P=0.850) (Table 3).
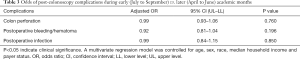
Full table
Discussion
As far as we know, this is the first large nationwide study looking for the existence of a July effect in the all-cause of mortality, the incidence of complications, LOS, total hospital charges and the disposition of patients following colonoscopy procedures at urban-teaching settings. We have also compared the odds of post-colonoscopy complications between early (July to September) vs. later (April to June) academic months. The noteworthy result of this study is that July effect was not identified in the utilization and the outcomes of colonoscopy procedure in the urban teaching hospitals. Our results indicate that experienced gastroenterologists monitor fellows closely and share their skills, experiences and knowledge with them while performing the procedure to improve the potential complications and procedure-related outcomes.
Not many studies have compared the outcomes of only gastroenterologist (GI) physicians involved in the colonoscopy procedures vs. GI physicians with fellows or trainees involved colonoscopy procedures. The higher risk of colonic perforation was documented with GI fellows and untrained physician during the procedure (27,28). Anderson et al. investigated the incidence of colonic perforation from the colonoscopy and sigmoidoscopy procedure. Out of 10,486 colonoscopies procedures, authors found 20 colonic perforations. Among them, 8 colonic perforations happened when the procedures were performed by GI fellows. However, the study concluded that the risk of colonic perforation was not statistically amplified with GI fellows or trainee involved in the procedure (28). Bielawska et al. also documented that the risk of colonic perforation was 2% higher with non-GI endoscopists (surgeons and unknown specialty endoscopists) as compared to experienced GI physicians involved procedures (27). Both studies documented the worse outcomes with unexperienced GI trainee or physicians as compared to the trained GI physicians. However, we have not revealed the statistically significant difference in the incidence of post-colonoscopy complications between the inexperienced GI fellows (early academic months) vs. experienced GI fellows (later academic months).
The incidence of the all-cause mortality (0.03%) following the colonoscopy procedure was measured in previously published studies (3). Colonoscopy-related deaths (0.007%) (5,29) have not frequently occurred as well as post-colonoscopy complications are also rare. Therefore, it could be less probability to have the statistically significant difference in the all-cause mortality following the colonoscopy procedure between early vs. later academic months. Our data have not revealed any significant difference in the all-cause of mortality between the early vs. late academic months too.
There are numerous studies, which have assessed the July effect on length of hospital stay and hospitalization charges for various medical conditions. However, results of these studies have not been consistent. Barry et al. did not find any differences in the intensive care unit (ICU) LOS after adjusting for the severity of illness over the period of an academic year (18). In the same way, July phenomenon was also not documented while assessing differences in the total charges and LOS between early vs. later academic months for any medical illness (30) as well as for ERCP-induced pancreatitis (23). Conversely, both total hospital charges and LOS for a wide range of internal medicine patients were significantly lower with experienced house staff (later academic months) as compared to inexperienced trainees (early academic months) (19). In our study cohort, the existence of the July effect in total hospital charges, LOS, and disposition of patients was also not established following colonoscopy procedures at the teaching institutions.
The most significant strength of our study is the large sample size. NIS is one of the largest databases in the US, thus, results of this study represent national level healthcare practice. Our study cohort is large enough to minimize the selection and participation biases which are mostly limited to the small studies. However, some limitations of this study need to be addressed. Gastroenterology fellowships might not be offered at all urban teaching hospitals, it could be one of the factors for overestimation or underestimation as well as dilution of our study results. Our study cohort was derived from the administrative database. Therefore, ICD-9 CM coding errors could be a possibility in our study. It is always not possible to measure the severity of post-colonoscopy complications based on ICD-9 CM codes. The process of randomization is not possible in all the retrospective studies. Hence, remnant confounding cannot be eliminated even though we adjusted the confound factors in our analysis. We could not evaluate the effects of other factors such as bowel preparation, cecum intubation time, withdrawal time, the degree of supervision by GI physicians. All these factors could be the quality indicators which could affect the study results. We could not differentiate between the screening colonoscopy and surveillance colonoscopy too. However, these limitations can be overlooked by strengths of the study.
Conclusions
The safety of colonoscopy was steady over the period of the academic year at urban teaching hospitals, as evidenced by no difference in the all-cause mortality and the incidence of post-colonoscopy complications between early and later academic months. The utilization and outcomes of colonoscopy procedures were also consistent across the academic year, as shown by no difference in LOS, total hospital charges and disposition of patients. Our study concludes that house staff experience might not influence the clinical outcomes in hospitalized patients undergoing colonoscopy. However, these results do not propose that just started GI fellowship trainees can perform colonoscopy procedure safely without the supervision of attending GI physician. These results also suggest that the GI fellows are being closely supervised by their GI attendings to develop the skills, techniques, and knowledge of colonoscopy procedures as per the current GI fellowship training guidelines.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fisher DA, Maple JT, Ben-Menachem T, et al. Complications of colonoscopy. Gastrointest Endosc 2011;74:745-52. [Crossref] [PubMed]
- Castro G, Azrak MF, Seeff LC, et al. Outpatient colonoscopy complications in the CDC's Colorectal Cancer Screening Demonstration Program: a prospective analysis. Cancer 2013;119:2849-54. [Crossref] [PubMed]
- Ko CW, Dominitz JA. Complications of colonoscopy: magnitude and management. Gastrointest Endosc Clin N Am 2010;20:659-71. [Crossref] [PubMed]
- Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009;150:849-57, W152.
- Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc 2001;53:620-7. [Crossref] [PubMed]
- Arora G, Mannalithara A, Singh G, et al. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc 2009;69:654-64. [Crossref] [PubMed]
- Korman LY, Overholt BF, Box T, et al. Perforation during colonoscopy in endoscopic ambulatory surgical centers. Gastrointest Endosc 2003;58:554-7. [Crossref] [PubMed]
- Nelson DB. Infectious disease complications of GI endoscopy: part II, exogenous infections. Gastrointest Endosc 2003;57:695-711. [Crossref] [PubMed]
- Kamath AS, Iqbal CW, Sarr MG, et al. Colonoscopic splenic injuries: incidence and management. J Gastrointest Surg 2009;13:2136-40. [Crossref] [PubMed]
- Sewitch MJ, Jiang M, Joseph L, et al. Rate of serious complications of colonoscopy in Quebec. Can J Gastroenterol 2012;26:611-3. [Crossref] [PubMed]
- Haller G, Myles PS, Taffe P, et al. Rate of undesirable events at beginning of academic year: retrospective cohort study. BMJ 2009;339:b3974. [Crossref] [PubMed]
- Barzansky B, Etzel SI. Medical schools in the United States, 2007-2008. JAMA 2008;300:1221-7. [Crossref] [PubMed]
- Young JQ, Ranji SR, Wachter RM, et al. "July effect": impact of the academic year-end changeover on patient outcomes: a systematic review. Ann Intern Med 2011;155:309-15. [Crossref] [PubMed]
- Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a "July effect"? Am J Orthop (Belle Mead NJ) 2009;38:606-11. [PubMed]
- Shuhaiber JH, Goldsmith K, Nashef SA. Impact of cardiothoracic resident turnover on mortality after cardiac surgery: a dynamic human factor. Ann Thorac Surg 2008;86:123-30; discussion 130-1. [Crossref] [PubMed]
- Jen MH, Bottle A, Majeed A, et al. Early in-hospital mortality following trainee doctors' first day at work. PLoS One 2009;4:e7103. [Crossref] [PubMed]
- Phillips DP, Barker GE. A July spike in fatal medication errors: a possible effect of new medical residents. J Gen Intern Med 2010;25:774-9. [Crossref] [PubMed]
- Barry WA, Rosenthal GE. Is there a July phenomenon? The effect of July admission on intensive care mortality and length of stay in teaching hospitals. J Gen Intern Med 2003;18:639-45. [Crossref] [PubMed]
- Rich EC, Gifford G, Luxenberg M, et al. The relationship of house staff experience to the cost and quality of inpatient care. JAMA 1990;263:953-7. [Crossref] [PubMed]
- Bakaeen FG, Huh J, LeMaire SA, et al. The July effect: impact of the beginning of the academic cycle on cardiac surgical outcomes in a cohort of 70,616 patients. Ann Thorac Surg 2009;88:70-5. [Crossref] [PubMed]
- Dhaliwal AS, Chu D, Deswal A, et al. The July effect and cardiac surgery: the effect of the beginning of the academic cycle on outcomes. Am J Surg 2008;196:720-5. [Crossref] [PubMed]
- Rich EC, Hillson SD, Dowd B, et al. Specialty differences in the 'July Phenomenon' for Twin Cities teaching hospitals. Med Care 1993;31:73-83. [Crossref] [PubMed]
- Schulman AR, Abougergi MS, Thompson CC. Assessment of the July effect in post-endoscopic retrograde cholangiopancreatography pancreatitis: Nationwide Inpatient Sample. World J Gastrointest Endosc 2017;9:296-303. [Crossref] [PubMed]
- Healthcare Cost and Utilization Project (HCUP) Database. Overview of the National (Nationwide) Inpatient Sample (NIS). Agency for Healthcare Research and Quality, Rockville, MD, 2018. Available online: https://www.hcup-us.ahrq.gov/nisoverview.jsp
- Obi K, Hinton A, Sobotka L, et al. Early Sigmoidoscopy or Colonoscopy Is Associated With Improved Hospital Outcomes in Ulcerative Colitis-Related Hospitalization. Clin transl gastroenterol 2016;7:e203. [Crossref] [PubMed]
- Navaneethan U, Parasa S, Venkatesh PG, et al. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. J Crohns & Colitis 2011;5:189-95. [Crossref] [PubMed]
- Bielawska B, Day AG, Lieberman DA, et al. Risk factors for early colonoscopic perforation include non-gastroenterologist endoscopists: a multivariable analysis. Clin Gastroenterol Hepatol 2014;12:85-92. [Crossref] [PubMed]
- Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol 2000;95:3418-22. [Crossref] [PubMed]
- Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology 2008;135:1899-906, 1906.e1.
- Buchwald D, Komaroff AL, Cook EF, et al. Indirect costs for medical education. Is there a July phenomenon? Arch Intern Med 1989;149:765-8. [Crossref] [PubMed]
Cite this article as: Desai R, Patel U, Goyal H. Does “July effect” exist in colonoscopies performed at teaching hospitals? Transl Gastroenterol Hepatol 2018;3:28.


