Differential diagnosis of gastrointestinal stromal tumor by histopathology and immunohistochemistry
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the human gastrointestinal (GI) tract. They can develop anywhere in the GI tract from the esophagus to the rectum. Approximately 60% of them arise in the stomach and approximately 30% in the small intestine. Duodenal and rectal GISTs account for about 5% each among whole GIST cases, and esophageal GISTs are rarer and colonic GISTs are extremely unusual. GISTs are considered to be derived from interstitial cells of Cajal (ICCs), which are spindle-shaped mesenchymal cells (1). Thus, GISTs are also typically composed of spindle cells. However, a minor proportion (10–15%) of them have epithelioid feature, and some tumors show mixed cell type. Histological diagnosis of GISTs should be considered according to the histological tumor cell type. For spindle cell type GISTs (Figure 1), spindle cell tumors such as desmoids, solitary fibrous tumors (SFTs), inflammatory myofibroblastic tumors (IMTs), perivascular epithelioid cell tumors (PEComas), inflammatory fibroid polyps (IFPs) are subject to the differential diagnoses. On the other hand, epithelioid cell tumors such as PEComas, carcinoids [or neuroendocrine tumors (NETs)] and adenocarcinomas should be distinguished from epithelioid type GISTs (Figure 2). Immunohistochemistry (IHC) is essential for the differential diagnoses of them. Antibodies used for IHC include those for KIT, desmin, S100 protein, α-smooth muscle actin (α-SMA), CD34, DOG1 (discovered on GIST-1), signal transducer and activator of transcription 6 (STAT6), β-catenin, and anaplastic lymphoma kinase (ALK). IHC for succinate dehydrogenase subunit B (SDHB) is needed for subtyping of GISTs, and Ki-67 IHC is useful for risk evaluation of GIST recurrence. Characteristics of these molecules used for IHC are described, and the above-mentioned tumors to be distinguished from GISTs are discussed here.
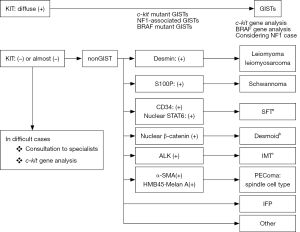
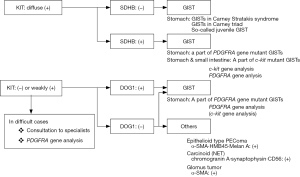
Characteristics of markers for IHC used for differentiating GISTs from other tumors
KIT
KIT, a receptor tyrosine kinase (RTK), is encoded by the protooncogene c-kit which is the cellular homolog of the v-kit oncogene (2). KIT is expressed by erythroblasts, melanocytes, germ cells, mast cells and ICCs, and its tyrosine kinase activity is important for development and proliferation of these five lineage cells. Since GISTs are considered to originate from ICC (1), most GISTs diffusely and strongly express KIT. Tumors other than GISTs developing in the GI tract are usually negative for KIT, but some tumors such as PEComas and melanomas might be positive for KIT. Some mutations of the c-kit gene result in loss-of-function of KIT tyrosine kinase activity, while other mutations of the c-kit gene cause constitutive activation of KIT tyrosine kinase. These gain-of-function mutations of the c-kit gene are observed in GISTs (1), mastocytomas (3), seminomas (4) and melanomas (5).
Desmin
Desmin is a muscle-specific protein and is one of the intermediate filaments constituting the cytoskeleton. In normal tissue, not only smooth muscle cells but also striated muscle cells are positive for desmin. Therefore, leiomyomas are basically positive for desmin. Leiomyosarcomas which might not show sufficient differentiation to smooth muscle cells are variably positive for desmin. On the other hand, GISTs and neurogenic tumors are commonly negative for desmin.
S100 protein
S100 proteins are a family of the calcium-binding proteins, and commercially available antibodies against S100 proteins mainly detect S100A. In normal tissues, S100A is present in Schwann cells, melanocytes, chondrocytes, adipocytes, myoepithelial cells, and so on. Among the GI mesenchymal tumors, almost all Schwannomas are positive for S100 protein. In contrast, almost all GISTs and smooth muscle cell tumors are negative for S100 protein.
α-SMA
Polymer of actin forms actin filament, one of the microfilaments. α-SMA, one of the six actin isoforms, is a major cytoskeletal structural network of smooth muscle cells, myofibroblasts and myoepithelial cells. It is positive for almost all smooth muscle cell tumors such as leiomyomas and leiomyosarcomas, almost all glomus tumors, most of IMTs and IFPs among GI mesenchymal tumors. We should be careful that a considerable number of GISTs, especially small intestinal GISTs, are also positive for this marker.
CD34
CD34 is a cell surface glycoprotein which plays a role in cell-cell adhesion. Immunohistochemically, CD34 is positive for hematopoietic stem cells, mesenchymal stem cells, endothelial progenitor cells and endothelial cells. Over 90% of gastric GISTs are positive for CD34, but approximately half of GISTs other than gastric GISTs are negative for CD34. A considerable number of IFPs are positive for CD34, and some of other GI mesenchymal tumors such as Schwannomas and leiomyomas might show partial and/or weak staining for CD34.
DOG1
DOG1 gene was found as a specific transcript in GISTs by using cDNA microarrays (6). DOG1 is a transmembrane calcium-activated chloride channel protein. Slow waves developing in the GI tract are considered to be associated with function of DOG1 in ICCs. DOG1 is normally expressed not only by ICCs but also by other cell types including acinar cells of the pancreas and salivary glands. Tumors such as renal oncocytomas, chondroblastomas, acinic cell carcinomas, chromophobe renal cell carcinomas are reported to be immunohistochemically positive for DOG1 in over 80% of those cases. Some other tumor types including adenoid cystic carcinomas, glomus tumors, synovial sarcomas also might show positive result for DOG1 IHC. Thus, DOG1 immunopositivity is not considered to be specific for GISTs (7).
STAT6
STAT6 is one of seven STAT family proteins. STAT6 is expressed in many tissues, especially in the immune system. Cytokine binding to the receptor induces the Janus kinase (JAK) activation, and subsequent STAT6 activation and homodimerization result in nuclear transfer of the protein. The nuclear STAT6 regulates (activates) the translation of the target proteins. In SFTs, NGFI-A binding protein-2 (NAB2)-STAT6 fusion protein is specifically detected in the nucleus (8,9).
β-catenin
Intracellular domain of the cadherins which play an important role in cell-cell adhesion binds to β-catenin, and the β-catenin can link cadherins with actin filaments. Thus, the β-catenin is important for maintenance of tissue structure. On the other hand, the β-catenin also plays a role as a factor for regulation of transcription. When Wnt signaling cascade is activated, stabilized non-phosphorylated β-catenin is transferred to nucleus to activate transcription of target genes. Mutations of the β-catenin gene also result in abundant accumulation of nuclear β-catenin and induce abnormal cell proliferation. As observed in colorectal cancers and malignant melanomas, most desmoids have β-catenin gene mutations (10) and nuclear accumulation of abundant β-catenin protein.
ALK
ALK is a RTK which has a transmembrane domain and an extracellular domain. ALK tyrosine kinase activity plays an important role in the development of the central nervous system. ALK can function as an oncogenic molecule by formation of fusion genes with some other genes. Nucleophosmin (NPM) gene is a main partner in anaplastic large-cell lymphomas, EML-4 in adenocarcinomas of the lung, and TMP3/4 in IMTs (11).
SDHB
Succinate dehydrogenase (SDH) is composed of several components including subunit A (SDHA), SDHB, subunit C (SDHC) and subunit D (SDHD). SDH complex is fixed at the inner membrane of the mitochondria and plays an important role in both tricarboxylic acid (TCA) cycle and electron transport system. In the TCA cycle, SDH complex oxidizes succinate to fumarate. Mutations of every subunit of SDH result in unstable condition of SDH complex, and the degradation and inactivation of SDH complex induce accumulation of succinate. High concentration of succinate results in decreased hypoxia induced factor 1 (HIF-1) degradation, and the resultant high level of HIF-1 induce proliferation of tumor cells via vascular channel formation.
Ki-67
Ki-67 is a nuclear protein which is present in proliferating cells during G1, S, G2 and M phases. Ki-67 is used as a cell proliferation marker and is regarded as a predictor of tumor recurrence. High Ki-67 labelling index usually means high recurrence rate of the tumor. In clinical practice in GIST, Ki-67 IHC may be used for both the direct prediction of recurrence and the determination of hot spot (high mitotic area) for mitotic counting.
Histology, IHC and gene abnormalities in GIST subtypes and tumors to be distinguished from GISTs
GISTs, spindle cell type
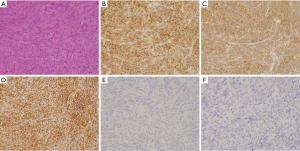
Basically, almost all spindle cell type GISTs express KIT strongly and diffusely (Figure 3A,B). Thus, the diagnosis of spindle cell type GISTs is not difficult. DOG1 is also expressed by almost all spindle cell type GISTs (Figure 3C). CD34 shows over 90% of gastric spindle cell type GISTs (Figure 3D), but about half of GISTs other than gastric origin do not apparently express CD34. Expression of CD34 appears to be inversely related to that of α-SMA. Thus, positive rate of α-SMA is not so high in gastric GISTs, but it is high in GISTs other than gastric GISTs. Expression of desmin (Figure 3E), S100 protein (Figure 3F), ALK, nuclear β-catenin and nuclear STAT6 is not usually detected. Ki-67 labelling index is variable from tumor to tumor. High Ki-67 labelling index and high mitotic counts predict high recurrence rate of GISTs. Representative genotypes of spindle cell type GISTs include c-kit gene mutants, NF1 mutants and BRAF mutants (Figure 1).
GISTs, epithelioid cell type
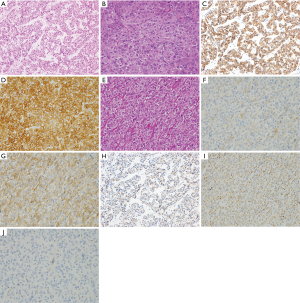
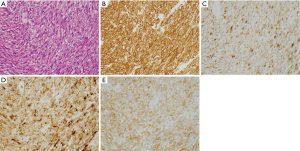
Representative genotypes of epithelioid cell type GISTs are c-kit gene mutants, Platelet-derived growth factor receptor alpha (PDGFRA) gene mutants and SDH gene abnormal types. About half of epithelioid cell type GISTs have c-kit gene mutations or SDH gene abnormalities. In these cases, KIT is expressed strongly and diffusely (Figure 4A-D). However, in residual half of epithelioid cell type GISTs such as PDGFRA gene mutants, expression of KIT is variable. Diagnosis of GISTs with low or almost no KIT expression may be difficult. As described above, KIT and DOG1 show very similar expression pattern. However, DOG1 may be more clearly positive in some PDGFRA mutant GISTs (Figure 4E-G), and KIT may be more clearly positive than DOG1 in other PDGFRA mutant GISTs. Thus, DOG1 IHC may be useful for the diagnosis of GISTs with low or almost no KIT expression. SDHB is positive in c-kit mutant GISTs (Figure 4H) and PDGFRA mutant GISTs (Figure 4I), while it is immunohistochemically negative in the SDH gene abnormal GISTs including those in Carney Stratakis syndrome (12), those in Carney triad and so-called juvenile (or pediatric) GISTs (Figure 4J) (13). About half of epithelioid type GISTs express CD34. Expression of desmin, S100 protein, ALK, nuclear β-catenin and nuclear STAT6 is not usually detected. Ki-67 labelling index is variable from tumor to tumor. High Ki-67 labelling index and high mitotic counts might predict high recurrence rate of the tumor as in the case of spindle cell type GISTs.
Leiomyomas
Leiomyomas are hypocellular tumors composed of spindle cells (Figure 5A). Almost all tumors are diffusely and strongly positive for α-SMA and desmin (Figure 5B) but not for KIT (Figure 5C), DOG1, S100 protein (Figure 5D), ALK, nuclear β-catenin and STAT6. CD34 is sometimes positive in leiomyomas. Although tumor cells themselves are not positive for KIT, many KIT-positive mast cells are often scattered within the tumor (Figure 5C). Similarly, leiomyomas often contain many ICCs that are positive for both KIT and DOG1. Thus, we should take care of evaluation of the IHC result of KIT and DOG1 in leiomyomas.
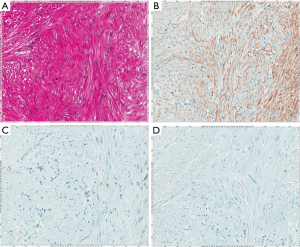
Leiomyosarcoma
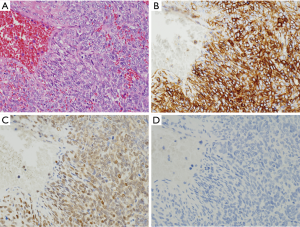
Leiomyosarcomas are hypercellular tumors composed of spindle cells (Figure 6A). The tumor cells of them are often more pleomorphic than those of GISTs, but the differential diagnosis between leiomyosarcomas and GISTs is not always easy only by histology on hematoxylin and eosin staining. Almost all tumors are diffusely and strongly positive for α-SMA (Figure 6B). Desmin might be also diffusely and strongly positive, but some tumors show partial and/or weak staining (Figure 6C). No apparent staining of desmin might be shown in some cases. Basically, they are negative for KIT (Figure 6D), DOG1, S100 protein, ALK, nuclear β-catenin and STAT6. CD34 is sometimes positive in leiomyosarcomas. Similarly, in leiomyomas, some leiomyosarcomas contain KIT-positive mast cells (Figure 6D) and KIT and DOG1-positive ICCs within the tumors.
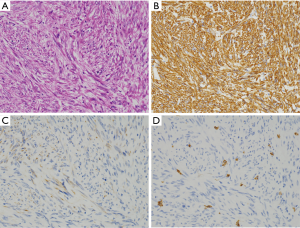
Schwannomas
Schwannomas are a spindle cell tumor. Density of the tumor cells (nuclei) may be high in parts, and that may be sparse in other parts (Figure 7A). Schwannomas of the GI tracts are often surrounded by foci of lymphocyte aggregation (Figure 7A). Almost all tumors are diffusely and strongly positive for S100 protein (Figure 7B). KIT (Figure 7C), DOG1, desmin (Figure 7D), α-SMA, ALK, nuclear β-catenin and STAT6 are basically negative. CD34 might be positive in some Schwannomas.
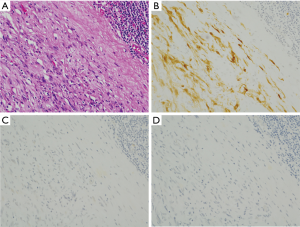
Desmoids
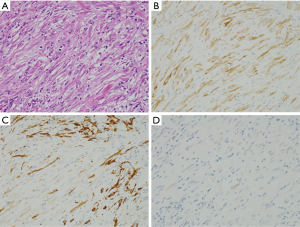
Desmoids are composed of spindle or stellate cells (Figure 8A). Tumors are usually paucicellular (Figure 8A). Most of them have mutations at exon 3 of the β-catenin gene. As described above, the mutations result in inhibition of degradation and subsequent accumulation of the protein. The increased protein is transferred to nuclei, and functions as transcription activator for the target proteins. Nuclear staining of β-catenin by IHC is observed in most of desmoids (Figure 8B). Basically, they are negative for KIT (Figure 8C), DOG1, S100 protein, ALK and STAT6. Desmoids might show non-specific weak staining by certain types of KIT antibodies using inappropriate staining procedure, but we can not observe diffuse and strong staining of KIT in desmoids as observed in GISTs. Thus, the β-catenin IHC is useful to diagnose desmoids accurately. Analysis of β-catenin gene mutation is a more direct method for β-catenin abnormality on diagnosis of desmoids (10).
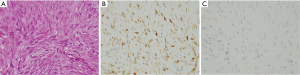
IFP
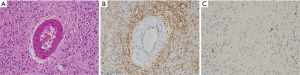
IFPs usually show a polyp-like finding protruding into the GI tract lumen. They are composed of sparse fibroblast-like spindle cells and often contain inflammatory cells especially eosinophils (Figure 9A). The fibroblastic spindle cells are often arranged concentrically around vessels (so-called onion-skin lesion) (Figure 9A). CD34 (Figure 9B) and α-SMA might be positive for the fibroblastic spindle cells in the tumors, but negative cases are also present. Basically, the spindle cells are negative for KIT (Figure 9C), DOG1, S100 protein, ALK, nuclear β-catenin and STAT6. Since mutations of PDGFRA gene mostly observed at exon 12 and rarely at exon 18 are detected in almost all IFP cases, IFPs are considered to be a genuine neoplasm (14).
PEComas
PEComas might show epithelioid cell type morphology or spindle cell type one (Figure 10A). They are usually positive for both smooth muscle markers such as α-SMA (Figure 10B) and melanoma markers such as HMB45 (Figure 10C), Melan A (Figure 10D) and MITF. KIT is apparently stained in some cases (Figure 10E). Basically, tumor cells are negative for DOG1, S100 protein, ALK, nuclear β-catenin and nuclear STAT6. TFE3 is positive in some cases by IHC, and rearrangement of TFE3 by fluorescent in situ hybridization (FISH) might be detected in those cases (15,16).
SFTs
SFTs are basically spindle cell tumors and have thick collagen bands and vessels with staghorn-like configuration (Figure 11A). Almost all tumors are positive for CD34 (Figure 11B) and nuclear STAT6 (Figure 11C). Basically, tumor cells are negative for KIT (Figure 11D), DOG1, S100 protein, ALK and nuclear β-catenin. They have NAB2-STAT6 fusion genes in most cases (8,9).
IMTs
IMTs often contain many inflammatory cells especially lymphocytes and plasma cells within the tumors (Figure 12A). Immunohistochemically, half of them are positive for ALK (Figure 12B). Rearrangement of ALK gene is detected by FISH in half of the cases (11). α-SMA is usually positive in IMTs (Figure 12C), but the tumor cells are basically negative for KIT (Figure 12D), DOG1, S100 protein, nuclear β-catenin and nuclear STAT6.
Glomus tumors
Most of glomus tumors composed of epithelioid tumor cells. In the GI tract, glomus tumors are very rare. Almost all glomus tumors are positive for α-SMA. However, they are usually negative for KIT, DOG1, CD34, desmin, S100 protein, nuclear β-catenin and nuclear STAT6.
Conclusions
GISTs, leiomyomas and Schwannomas are main GI mesenchymal tumors, but many other types of soft tissue tumors might develop in the GI tract. We have to be careful for the differential diagnoses of these GI mesenchymal tumors. Since we have expensive but very effective molecular target drugs such as imatinib, sunitinib and regorafenib for GIST treatment, not only incorrect diagnosis of GIST in nonGIST cases but also incorrect diagnosis of nonGIST in GIST ones give disadvantage to those patients. For correct diagnosis of GISTs, IHC and/or unique genetic analyses for differential diagnoses are necessary.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Besmer P, Murphy JE, George PC, et al. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature 1986;320:415-21. [Crossref] [PubMed]
- Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A 1995;92:10560-4. [Crossref] [PubMed]
- Tian Q, Frierson HF Jr, Krystal GW, et al. Activating c-kit gene mutations in human germ cell tumors. Am J Pathol 1999;154:1643-7. [Crossref] [PubMed]
- Willmore-Payne C, Holden JA, Tripp S, et al. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol 2005;36:486-93. [Crossref] [PubMed]
- West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165:107-13. [Crossref] [PubMed]
- Swalchick W, Shamekh R, Bui MM. Is DOG1 Immunoreactivity specific to gastrointestinal stromal tumor? Cancer Control 2015;22:498-504. [Crossref] [PubMed]
- Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet 2013;45:131-2. [Crossref] [PubMed]
- Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 2013;45:180-5. [Crossref] [PubMed]
- Miyoshi Y, Iwao K, Nawa G, et al. Frequent mutations in the beta-catenin gene in desmoid tumors from patients without familial adenomatous polyposis. Oncol Res 1998;10:591-4. [PubMed]
- Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol 2000;157:377-84. [Crossref] [PubMed]
- Pasini B, McWhinney SR, Bei T, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet 2008;16:79-88. [Crossref] [PubMed]
- Gill AJ, Chou A, Vilain R, et al. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am J Surg Pathol 2010;34:636-44. [PubMed]
- Schildhaus HU, Cavlar T, Binot E, et al. Inflammatory fibroid polyps harbour mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene. J Pathol 2008;216:176-82. [Crossref] [PubMed]
- Tanaka M, Kato K, Gomi K, et al. Perivascular epithelioid cell tumor with SFPQ/PSF-TFE3 gene fusion in a patient with advanced neuroblastoma. Am J Surg Pathol 2009;33:1416-20. [Crossref] [PubMed]
- Argani P, Aulmann S, Illei PB, et al. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol 2010;34:1395-406. [Crossref] [PubMed]
Cite this article as: Hirota S. Differential diagnosis of gastrointestinal stromal tumor by histopathology and immunohistochemistry. Transl Gastroenterol Hepatol 2018;3:27.


