Neoadjuvant therapy for gastrointestinal stromal tumor
Introduction
Adjuvant therapy is one of useful options for multidisciplinary treatment of advanced gastrointestinal tumors. The objective of adjuvant therapy is to control remnant micrometastases that may be left even after radical surgery, thereby suppressing recurrence and improving survival compared with that of surgery only. Neoadjuvant therapy is strictly a preoperative treatment for the purpose of improving survival in patients with resectable tumors, unlike treatment for the patients with unresectable/metastatic tumors. Since gastrointestinal surgery may change the state of oral intake greatly and decrease postoperative treatment tolerability, the role of neoadjuvant therapy is considered to be particularly important. For example, a randomized trial comparing postoperative and preoperative chemotherapy for localized advanced esophageal carcinoma revealed that the overall survival (OS) of the neoadjuvant group was better than that of the adjuvant group (1).
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor in the digestive tract. Patients with large tumor size and large mitotic count have a high risk of recurrence after surgery (2), and researches about neoadjuvant therapy are being conducted, similar to the case in gastrointestinal carcinoma. Most GISTs express KIT, a receptor tyrosine kinase encoded by proto-oncogene c-kit, and gain-of-function mutations of c-kit are a major cause of tumorigenesis and proliferation (3). Heretofore, tyrosine kinase inhibitors have been effective, and dramatic improvements have been seen in the prognosis of metastasis/recurrent GIST especially after the molecular-targeting therapeutic agent imatinib mesylate has been introduced into therapy (4).
In the neoadjuvant setting, it is expected that improvement of recurrence rate and survival rate can be obtained by imatinib therapy, which has already been proved to have a high clinical efficacy for metastasis/recurrent GIST. GIST usually shows expansive growth and is often found after the tumor is already quite large. For radical surgery, it may be necessary to sacrifice organ function or to require resection of other organs. Neoadjuvant therapy for large GISTs may have the potential to increase the complete resection rate by decreasing the tumor size and perhaps more importantly, to decrease the risk of surgical rupture or extended surgery. The aim of this article is to introduce previous evidence and strategies regarding neoadjuvant therapy for GIST.
Clinical trials
Although case reports on neoadjuvant imatinib therapy have been seen since 2003, the results of multicenter trials were first reported in 2009 (Table 1). Retrospective analyses focusing on neoadjuvant therapy were conducted from two large-scale clinical databases: the BFR14 trial (7), a phase III trial for interruption of imatinib in non-progressive patients and a database from ten centers of the European Organization for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) (11). The following three trials are representative phase II trials aimed at neoadjuvant imatinib therapy.
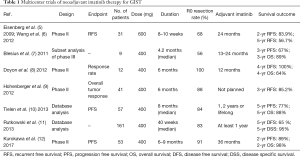
Full table
RTOG0132 trial
RTOG (Radiation Therapy Oncology Group) 0132 was the first trial of preoperative imatinib in GIST (5,6). It was a prospective phase II study of neoadjuvant/adjuvant imatinib mesylate for operable GIST cases registered in the United States from 2002 to 2006. Short-term and long-term results have been reported. The subjects were KIT-positive GIST patients with either primary disease (>5 cm) or metastatic/recurrent disease (>2 cm). Thirty-one primary GIST patients were analyzed as the neoadjuvant group. Sixteen (52%) were patients with GIST of the stomach and 4 (13%) had GIST of the small intestine. The median tumor size was 8.7 cm. Imatinib was administered at 600 mg/day for 8 to 12 weeks before surgery, and imatinib administration also continued for 2 years after surgery. For all 52 patients in the early report, the rates of grade 3, 4, and 5 preoperative toxicities were 21%, 12%, and 2%, respectively, and the median period of preoperative imatinib was 65 days. In the evaluation by RECIST, partial response (PR) was 7%, stable disease (SD) was 83%, and 21 of 31 patients (68%) underwent R0 resection. The rates of grade 3, 4, and 5 postoperative toxicities were 34%, 20.8%, and 1.9%, respectively. In the primary GIST group, the progression-free survival (PFS), which was the primary end point of this trial, was calculated as 83.9% for 2 years and 56.7% for 5 years. The 5-year OS was 76%. This trial demonstrated the feasibility of preoperative imatinib, but failed to demonstrate the superiority of adding neoadjuvant therapy compared with the results of adjuvant therapy alone.
APOLLON trial
The APOLLON trial was a prospective, phase II study of neoadjuvant imatinib for advanced GIST registered between 2005 and 2009 in Germany (9). The subjects were locally advanced, non-metastatic GIST cases, and there was no provision for tumor size. Forty-one patients with primary GIST were enrolled and the median tumor size was 10.8 cm. The preoperative dose of imatinib was 400 mg/day for 6 months, with an average of 200 days administered. Dose reduction or interruption due to toxicity was required in two patients. Surgical resection was performed in 34 cases, and R0 resection was undergone in 30 cases. Postoperative treatment was not planned in the study. The 3-year relapse-free survival (RFS) was 85.2%. Since this result did not depend on adjuvant therapy, the potential of neoadjuvant imatinib was expected.
Asian multinational phase II study
Between 2010 and 2014, a phase II study of neoadjuvant imatinib for large gastric GIST was conducted in Japan and South Korea. Its short-term results were recently reported (12). The 53 patients registered in this study had no previous treatment and primary gastric GIST ≥10 cm. The median tumor size was 12.0 cm. Prior to surgery, imatinib treatment was set at 400 mg daily for 6–9 months, and the median duration of neoadjuvant therapy was 26 weeks. The most frequent Grade 3–4 adverse events were rashes, at 9%, followed by neutropenia, at 8%. Although dose reduction of imatinib was required in 14 patients, 46 patients (87%) received preoperative administration for more than 6 months. The response rate by RECIST was 62%. Surgical resection was performed in 50 patients, and R0 excision was performed in 48 patients (91%). Furthermore, forty-two patients achieved preservation of at least half of the stomach. Forty patients received adjuvant imatinib and 38 of these continued imatinib therapy for at least 1 year after surgery. At the median follow-up time of 32 months, 2-year PFS and OS were 89% and 98%, respectively. This study showed that neoadjuvant imatinib for 6–9 months was feasible and brought about a high R0 resection rate. Long-term results are expected to provide improved evidence of the survival benefit of neoadjuvant imatinib for high-risk GISTs.
Selection of therapeutic agents
Generally, drugs used for neoadjuvant therapy are required to have high antitumor efficacy. Molecular targeting therapy using imatinib mesylate, which is the standard treatment for unresectable or metastatic/recurrent GISTs, would also be appropriate for agent of neoadjuvant. It does not necessarily have an excellent response rate by the RECIST criteria. The B2222 trial was a randomized Phase II study comparing imatinib at 400 and 600 mg/day for unresectable or metastatic GIST (13). The overall objective response rate was 68%, and 23 patients (16%) achieved SD. The estimated 5-year OS was 55%, equal in patients who achieved either SD or PR. The efficacy of molecular target therapy for GIST patients should be determined by the disease control rate (DCR), which is the sum of complete response (CR), PR, and SD. The DCR of imatinib therapy in various clinical studies of advanced GIST has been reported as 70–90% (13-16). The efficacy of imatinib therapy for advanced GIST is high in this regard, so imatinib therapy would be recommended also for neoadjuvant therapy.
The initial dose of imatinib of 400 mg/day is considered to be reasonable as a standard dose. In the early days of neoadjuvant imatinib therapy, high doses such as 600 and 800 mg/day were also examined, but no obvious superiority was observed compared to 400 mg/day (5,6,17). Demetri et al. examined the plasma level of imatinib mesylate and grouped patients into quartiles according to their imatinib trough concentration (18). The time to progression was equivalent among the three groups except for the lowest-concentration group (<1,100 ng/mL). This indicates that high-dose administration is not necessary in imatinib treatment if sufficient plasma concentration is obtained. Bouchet et al. also reported that the effectiveness is low when the imatinib plasma level is not sufficient, and a trough concentration of 760 ng/mL is required regardless of the primary organ (19). There are individual differences in the blood concentration of the drug, and it is recommended to investigate the imatinib trough level when performing neoadjuvant therapy. On the other hand, it had been confirmed that the high-dose imatinib administration have a PFS advantage on the therapy of unresectable/metastatic GISTs with KIT exon 9 mutations (20). It is also known that there are the imatinib-resistant GISTs such as the GISTs with wild-type KIT, platelet-derived growth factor receptor alpha (PDGFRA) D842V mutation and so on (21). The examination of the KIT/PGDFRA mutation status is recommended if the biopsy is possible before surgery.
Multikinase inhibitors such as sunitinib malate and regorafenib are also used as molecular targeted therapeutic agents for the treatment of GIST. These can be expected to be effective against imatinib-resistant GIST, and there are a few case reports in which neoadjuvant sunitinib therapy was conducted (22). However, these multi-kinase inhibitors have been implicated in various complications in surgery, such as hypertension, thrombosis, delayed wound healing and so on. Raut et al. reported that surgical morbidity after sunitinib administration was as high as 54% (23). Unlike imatinib therapy, the use of multikinase inhibitors in a neoadjuvant setting needs to be weighed carefully in terms of its potential advantages and risks.
Preoperative treatment period
There is not enough evidence about the appropriate treatment period of neoadjuvant imatinib therapy for advanced GIST. Raut et al. examined surgical cases in the state of stable disease, limited disease progression, and generalized disease progression after imatinib treatment (24). Twelve-month progression-free survival was 80% for patients with SD, better than 33% for those with limited progression and 0% for those with generalized progression. The authors concluded that surgery has little to offer in the setting of generalized progression while surgery for patients with disease control during imatinib therapy is meaningful. Mussi et al. also reported the surgical outcomes of 80 patients with metastatic GIST after imatinib treatment (25). The survival outcome of surgery for patients at the time of best clinical response was better than that of focal progression (2-year PFS, 64% versus 10%; 5-year disease specific survival, 83% versus 68%). From these results, it is recommended that surgery on patients treated with imatinib mesylate should be timed to coincide with the best clinical response.
The pharmacological effect of imatinib therapy is promptly expressed, but it takes time to decrease tumor size because imatinib works as a cytostatic agent. Therefore, imatinib needs to be administered for longer periods than the usual neoadjuvant chemotherapies for carcinoma. In the B2222 trial it was reported that the median time to the response of patients who gained effects higher than PR was 2.7 months, and it took 5.3 months for 75% of patients to get a response (26). Tirumani et al. reported that best response to neoadjuvant imatinib was seen at 28 weeks and plateau response was seen at 34 weeks (16). From these results, it seems that the neoadjuvant treatment period of 2 to 3 months established in the RTOG 0132 trial was too short for imatinib treatment to exert a beneficial decrease in tumor size. In order to obtain sufficient cytoreductive or cytocidal effect, imatinib should be administered for at least 6 months prior to surgery.
On the other hand, too long a treatment also has risks. Surgery should also be performed before drug resistance to imatinib occurs. In the B2222 trial, half of the patients had tumor progression within 2 years after starting imatinib administration (15). The median time to progression in patients with stable disease was 12 months. Surgical intervention after disease progression should be avoided, and surgery should be considered cautiously if imatinib treatment has been carried out for more than 1 year.
Postoperative therapy
Although it must be carefully considered whether neoadjuvant therapy should be performed on GIST patients who have a high risk of recurrence, adjuvant imatinib therapy after curative surgery is standard treatment for these high-risk patients. Rutkowski et al. analyzed data of 161 GIST patients who received neoadjuvant imatinib therapy in EORTC-STBSG (11). One year or more of adjuvant imatinib therapy was conducted in 91 patients (57%), and the median period of adjuvant imatinib administration was 40 weeks. Among patients who received adjuvant therapy, the five-year DFS was 72%, better than that of the 70 patients who did not receive postoperative treatment, 57%. Even after neoadjuvant therapy, postoperative adjuvant imatinib therapy is considered essential.
The SSG XVIII/AIO trial, a randomized phase III study, compared the 1-year versus 3-year administration of adjuvant imatinib in the treatment of high-risk GIST patients (27). In the 3-year treatment group, the five-year RFS was 65.6%, better than the 47.9% in the 1-year treatment group. In addition, the five-year OS was also better in the 3-year treatment group (92% versus 81.7%). This study demonstrated that adjuvant imatinib therapy improves the prognosis of high risk GIST. Recently, the results of a single-arm, phase II trial of 5-year administration of adjuvant imatinib were reported (PERCIST-5 trial) (28). The long-term survival was good: the 8-year RFS was 81% and 8-year OS was 95%, respectively. Although an appropriate period is not clear in postoperative treatment for patients after neoadjuvant imatinib, at least 3 to 5 years’ administration seems to be needed as with simple adjuvant imatinib therapy.
Prevention of extended surgery
GIST develops in any part of the gastrointestinal tract from the esophagus to the rectum, but has a high incidence in the stomach (60%) and the small intestine (30%). Lymph node metastasis is rarely seen, so lymph node dissection and extensive excision of associated organs is unnecessary in contrast to the radical surgery for gastrointestinal carcinoma (29). However, GIST often shows expansive development, and is often diagnosed after experiencing an increase in size without defined subjective symptoms such as obstruction, bleeding and pain. Therefore, the range of organ resection may be enlarged or multiple organ involvement may be necessary for resection of large tumors. For this reason, preoperative treatment is also expected to be favored from the viewpoint of organ/function preservation by tumor shrinkage.
The most commonly reported treatment for organ preservation is rectal primary GIST. Although rectal GIST is uncommon, only about 5% of all GIST, it becomes a problem as to whether the anus can be preserved in order to secure a sufficient margin. Wilkinson et al. reported 15 patients with rectal GIST who received neoadjuvant imatinib therapy, and nine of these patients underwent surgery (30). Neoadjuvant therapy enabled sphincter-preserving surgery to be undertaken in seven patients who would have otherwise required abdominoperineal resection or pelvic exenteration. Pai et al. reported a retrospective analysis of rectal GIST (31). Only 3 of 9 patients were able to preserve the sphincter despite the fact that the DCR was 92% including 54% partial response. Although the efficacy for quality of life is great if neoadjuvant imatinib can preserve the anal sphincter and avoid an ostomy, it should be noted that the clinical situations such as tumor localization or other factors can make this difficult.
In the case of duodenal GIST, the pancreas is adjacent, and combined resection may be necessary. Lv et al. reported that neoadjuvant imatinib administration was performed on ten locally advanced duodenal GIST patients in whom nine were deemed eligible for pancreatic preservation surgery (32). To avoid postoperative pancreatitis or pancreatic fistula, neoadjuvant imatinib for patients with large duodenal GIST may be considered. In the case of gastric GIST, neoadjuvant imatinib has been reported to be helpful for avoiding total gastrectomy (12,33). There is also the merit of making laparoscopic radical surgery possible by reducing the size of the tumor (34). Although there are few reports about GIST of the esophagus or esophagogastric junction, neoadjuvant imatinib may have the potential to eliminate the need for a transthoracic approach at curative resection (35-37).
Research on neoadjuvant imatinib aiming at organ preservation is still insufficient. It should make sure the period of neoadjuvant therapy does not become unnecessarily long by seeking too great a decrease in tumor size; the timing of the best response should not be missed.
Conclusions
The importance of neoadjuvant treatment lies in its feasibility and its survival outcome. The feasibility of neoadjuvant imatinib therapy seems to be well established from the results of clinical trials. However, proof of the survival effectiveness of neoadjuvant-setting imatinib therapy has not been sufficiently demonstrated. It is expected that the long-term results of phase II study for large gastric GIST in Japan and South Korea will prove the survival benefit of neoadjuvant imatinib therapy. Clinical questions still remain about the most appropriate period of pre- and post-operative imatinib administration in the neoadjuvant protocol. The benefits of neoadjuvant therapy with other tyrosine kinase inhibitors against imatinib-resistant GIST are also controversial. Since GIST is a rare disease and cases are limited, neoadjuvant therapy should be registered in nationwide or worldwide clinical trials/databases to compile meaningful bodies of evidence.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Rutkowski P, Debiec-Rychter M, Nowecki ZI, et al. Different factors are responsible for predicting relapses after primary tumors resection and for imatinib treatment outcomes in gastrointestinal stromal tumors. Med Sci Monit 2007;13:CR515-22. [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer 2003;39:2006-11. [Crossref] [PubMed]
- Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 2009;99:42-7. [Crossref] [PubMed]
- Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 2012;19:1074-80. [Crossref] [PubMed]
- Blesius A, Cassier PA, Bertucci F, et al. Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer 2011;11:72. [Crossref] [PubMed]
- Doyon C, Sidéris L, Leblanc G, et al. Prolonged Therapy with Imatinib Mesylate before Surgery for Advanced Gastrointestinal Stromal Tumor Results of a Phase II Trial. Int J Surg Oncol 2012;2012:761576. [Crossref] [PubMed]
- Hohenberger P, Langer C, Wendtner CM, et al. Neoadjuvant treatment of locally advanced GIST: results of APOLLON, a prospective, open label phase II study in KIT- or PDGFRA-positive tumors. J Clin Oncol 2012;30:abstr 10031.
- Tielen R, Verhoef C, van Coevorden F, et al. Surgical treatment of locally advanced, non-metastatic, gastrointestinal stromal tumours after treatment with imatinib. Eur J Surg Oncol 2013;39:150-5. [Crossref] [PubMed]
- Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20:2937-43. [Crossref] [PubMed]
- Kurokawa Y, Yang HK, Cho H, et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer 2017;117:25-32. [Crossref] [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [Crossref] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [Crossref] [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [Crossref] [PubMed]
- Nishida T, Shirao K, Sawaki A, et al. Efficacy and safety profile of imatinib mesylate (ST1571) in Japanese patients with advanced gastrointestinal stromal tumors: a phase II study (STI571B1202). Int J Clin Oncol 2008;13:244-51. [Crossref] [PubMed]
- McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16:910-9. [Crossref] [PubMed]
- Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol 2009;27:3141-7. [Crossref] [PubMed]
- Bouchet S, Poulette S, Titier K, et al. Relationship between imatinib trough concentration and outcomes in the treatment of advanced gastrointestinal stromal tumours in a real-life setting. Eur J Cancer 2016;57:31-8. [Crossref] [PubMed]
- Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [Crossref] [PubMed]
- Svetlichnaya J, Huyck TK, Wayne JD, et al. Neoadjuvant use of sunitinib in locally advanced GIST with intolerance to imatinib. Chemotherapy 2012;58:30-3. [Crossref] [PubMed]
- Raut CP, Wang Q, Manola J, et al. Cytoreductive surgery in patients with metastatic gastrointestinal stromal tumor treated with sunitinib malate. Ann Surg Oncol 2010;17:407-15. [Crossref] [PubMed]
- Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006;24:2325-31. [Crossref] [PubMed]
- Mussi C, Ronellenfitsch U, Jakob J, et al. Post-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients? Ann Oncol 2010;21:403-8. [Crossref] [PubMed]
- Tirumani SH, Shinagare AB, Jagannathan JP, et al. Radiologic assessment of earliest, best, and plateau response of gastrointestinal stromal tumors to neoadjuvant imatinib prior to successful surgical resection. Eur J Surg Oncol 2014;40:420-8. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Raut CP, Espat NJ, Maki RG, et al. Extended treatment with adjuvant imatinib (IM) for patients (pts) with high-risk primary gastrointestinal stromal tumor (GIST): The PERSIST-5 study. J Clin Oncol 2017;35:abstr 11009.
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Wilkinson MJ, Fitzgerald JE, Strauss DC, et al. Surgical treatment of gastrointestinal stromal tumour of the rectum in the era of imatinib. Br J Surg 2015;102:965-71. [Crossref] [PubMed]
- Pai VD, Demenezes JL, Patil PS, et al. Multimodality therapy of rectal gastrointestinal stromal tumors in the era of imatinib-an Indian series. J Gastrointest Oncol 2016;7:262-8. [PubMed]
- Lv A, Qian H, Qiu H, et al. Organ-preserving surgery for locally advanced duodenal gastrointestinal stromal tumor after neoadjuvant treatment. Biosci Trends 2017;11:483-9. [Crossref] [PubMed]
- Annaberdyev S, Gibbons J, Hardacre JM. Dramatic response of a gastrointestinal stromal tumor to neoadjuvant imatinib therapy. World J Surg Oncol 2009;7:30. [Crossref] [PubMed]
- Cavaliere D, Vagliasindi A, Mura G, et al. Downstaging of a gastric GIST by neoadjuvant imatinib and endoscopic assisted laparoscopic resection. Eur J Surg Oncol 2007;33:1044-6. [Crossref] [PubMed]
- Duffaud F, Meeus P, Bertucci F, et al. Patterns of care and clinical outcomes in primary oesophageal gastrointestinal stromal tumours (GIST): A retrospective study of the French Sarcoma Group (FSG). Eur J Surg Oncol 2017;43:1110-6. [Crossref] [PubMed]
- Sato H, Kanda T, Hirota S, et al. Surgical resection of gastrointestinal stromal tumor of esophagus following preoperative imatinib treatment: a case report. Esophagus 2010;7:65. [Crossref]
- Staiger WI, Ronellenfitsch U, Kaehler G, et al. The Merendino procedure following preoperative imatinib mesylate for locally advanced gastrointestinal stromal tumor of the esophagogastric junction. World J Surg Oncol 2008;6:37. [Crossref] [PubMed]
Cite this article as: Ishikawa T, Kanda T, Kameyama H, Wakai T. Neoadjuvant therapy for gastrointestinal stromal tumor. Transl Gastroenterol Hepatol 2018;3:3.


