Surgery of the pancreatic cystic echinococcosis: systematic review
Cystic echinococcosis (CE) is still a continuing health problem in the world (1). CE is a parasitic disease caused by the tapeworm Echinococcus granulosus. The tapeworm lives usually in the intestine of dog, called the “definitive host”. The tapeworm eggs are passed in the feces and are ingested by sheep, called the “intermediate host”. The eggs penetrate the intestinal wall of the sheep, and reach the liver using the portal vein, where they develop into a cyst. Afterward, in particular circumstances, they passed through the liver and reach all other viscera. Echinococcus infestation occurs in humans when they accidentally ingest tapeworm eggs (1).
After 30 years [1977–2005], the average annual surgical incidence rate slightly dropped, from 15 to 12.6/100,000 inhabitants/year, proving that this zoonosis remains a problem of public health in Tunisia (2). CE is localized in the liver for 60% to 70% (1). CE can be disseminated to other viscera such as pancreas owing to hematogenous contamination.
In 1975, Kattan (3) reported a prevalence of 0.25%: two PCE out of 780 cases of CE affecting various organs operated on in one surgical unit in Baghdad during the period 1963–1975. In Banat (central of Europe) (4), an epidemiological study was performed on 505 adults, CE was rarely found in pancreas (0.2%). The plain radiographs, ultrasound images and computed tomography (CT) scans of 357 CE seen between 1978 and 1998 were reviewed by Dahniya et al. (5). One PCE was reported with a prevalence of 0.3%. In the east of Azerbaijan (6), prevalence of PCE is 0.6%. Of 265 extra pulmonary hydatid cysts collected from 1990 to 2007, Bellil et al. found one CE located in the pancreas corresponding to 0.4% (7). Finally, the prevalence of PCE in the world ranges between 0.2% and 0.6%.
The diagnosis of PCE is easy when it is associated to other location such as liver, it became difficult when PCE was isolated simulating other diagnosis such as pseudocyst, a choledochal cyst, serous or mucinous cystadenoma and cystadenocarcinoma. There is no consensus concerning surgical management in medical literature. This systematic review aimed to provide evidence based answer to the following questions: (I) what are the efficient tools to affirm the diagnosis of isolated PCE? and (II) what are the best therapeutic strategy for the PCE?
Methods
An electronic search in several medical databases was performed by two authors (W Dougaz, I Bouasker). Medline, Scopus, Embase, Web of Science, Google Scholar and Cochrane collaboration were consulted. The keywords used were “cyst”, “echinococcosis”, “hydatid cyst” and “pancreas”. All abstracts were analyzed followed by extraction of the full text by the same two authors (W Dougaz, I Bouasker) and all divergences were resolved by discussion with C Dziri.
All full text of original articles, case report, and small series, reporting isolated PCE, with limitations to English, French, Spanish and Italian languages, were retained by W Dougaz and I Bouasker. Assessment of methodology (W Dougaz, I Bouasker) was to check that every full text should contain demographic data, complaints, physical examination, and cyst location, hydatid serology, imaging features, operative findings, operative procedures and postoperative course. All divergences were resolved by discussion with C Dziri. As concerns operative procedures and according to the expert consensus published in 2010 (8), we considered that radical surgery aims to remove cysts completely “total cystectomy”: laminated and germinal layers with or without adventitial layer called “pericyst”. Therefore, “total cystectomy” for the PCE included: closed or open total pericystectomy with or without resection of the pancreas and enucleation. On the other hand, we considered conservative surgery as “partial cystectomy”, in which the cyst content is sterilized and removed after opening, with the pericyst partially resected which is called “unroofing procedure” for others (8). We also considered conservative surgery all anastomosis between cysts and digestive tract and mini invasive surgery or percutaneous treatments (percutaneous drainage or PAIR technique which consisted to perform a puncture, aspiration, and injection of protoscolicid solution followed by re-aspiration) (8).
We excluded articles reporting PCE associated to cysts localized in other abdominal organs, literature reviews and editorials.
A descriptive analysis was performed. Qualitative variables were mentioned with its percentage, quantitative variables were reported with their mean value ± standard deviation when the distribution is normal otherwise with median value and interquartile range. Recommendations were based on Oxford’s classification (9).
Results
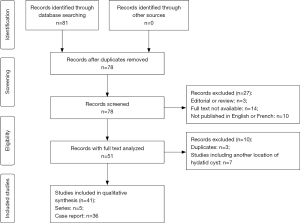
We retrieved 81 articles from 2001 to 2017, 41 articles (10-30) were retained (31-50) (Figure 1), published in 30 journals according to the eligibility criteria. Sixty-two patients were enrolled in this systematic review, extracted from 36 articles of cases report and five articles reporting small series. This sample included 37 women and 25 men living in Mediterranean countries, South Africa, Iran and India. The mean age was 34.76±18.03 years [median age, 32.00 years; interquartile ranges (IQR), 20.00–48.50 years; range: 4.00–72.00 years]. The main symptom was pain localized in the epigastria (69%) or in the upper left quadrant (31%). Pain was associated to vomiting in 16%, jaundice in 26% owing to a compression of the common bile duct and fever in 8%. Acute pancreatitis may reveal a PCE in 15%.
Physical examination showed an abdominal mass in 32%. Ultra-sonography (US) and CT scan were the most performed exam respectively in 71% in 90%. Hydatid serology is not very helpful, it has a low sensitivity 62%. Cyst was localized in the head (31%), in the body (22%) and in the tail (47%). PCE compressed the common bile duct in 16 patients who had cyst in the head (26%). Cyst size was reported in 49 patients out of 62, the median diameter was 70 mm (IQR, 41–100 mm and range, 24–180 mm).
Open approach was performed in 95% and laparoscopic approach in 5%. Radical surgery was performed in 32 patients (52%): five duodenopancreatectomies (8%), one central pancreatectomy, 20 distal pancreatectomies (32%) associated to splenectomy in 16 cases. Six patients underwent pericystectomy. A conservative surgery was performed in 30 cases (48%): 27 unroofing procedures, one cystic digestive anastomosis and two patients had percutaneous drainage associated to injection of albendazole in the cystic cavity.
On the other hand, in this sample of 62 patients, the authors have different surgical indication according to the location of the cyst:
- Head (n:19): nine radical surgery (five duodenopancreatectomies + two total resections + two enucleations) versus 10 conservative surgery (nine unroofing + one cystic digestive anastomosis);
- Body (n:14): five radical surgery (two total resections + one central pancreatectomy + two distal pancreatectomies) versus nine conservative surgery (eight unroofing+ one percutaneous drainage associated to injection of albendazole in the cystic cavity);
- Tail (n:29): 18 radical surgery (18 distal pancreatectomies) versus 11 conservative surgery (10 unroofing + one percutaneous drainage associated to injection of albendazole in the cystic cavity).
No death occurred in this sample. Postoperative morbidity included five pancreatic fistulas (one post-duodenopancreatectomy + one post-distal pancreatectomy + three post-unroofing), two deep surgical site infections post-duodenopancreatectomy, and one bleeding post-duodenopancreatectomy. The follow-up was mentioned for 48 patients out of 62 (77%). Median of follow-up was 11 months (IQR, 4.25–24.00 months and range values, 0–132 months). One recurrence was reported.
Discussion
PCE is a rare localization with a prevalence ranging between 0.20% and 0.60%.
Clinical presentation
This systematic review showed that the main symptom was pain localized in the epigastria (69%) or in the upper left quadrant (31%), associated to vomiting in 16%, jaundice in 26% and fever in 8%. Acute pancreatitis may reveal a PCE in 15%.
Other authors reported cases with different clinical presentation. In 1957, Lebon et al. reported a case of jaundice owing to a compressive PCE of the head (51). Morton reported also an obstructive jaundice caused by an intrapancreatic hydatid cyst (52). Mzali et al. reported a PCE of the head ruptured in the common bile duct responsible of jaundice (53). In 1955, Joske (54) reported a case of a 44-year-old man who consulted for obstructive jaundice, a hydatid cyst of the head of the pancreas pressing upon the common bile duct was found. The cyst was removed and the patient developed pancreatic insufficiency and diffuse calcification of the pancreas some years later. Katkhouda et al. (55) reported a PCE of the tail responsible for chronic recurrent pancreatitis which raised diagnostic problems. Chinya et al. (18) described a hydatid cyst of the pancreas masquerading as pancreatic pseudocyst. On the other hand, PCE may mimic a cystic tumor during intervention such as a mucinous cystic neoplasia (56).
Hydatid serology
In this systemic review, hydatid serology has a low sensitivity of 62%. In the literature review of Akbulut (57), sensitivity of hydatid serology was also low (54%).
Imaging features
This systematic review showed that US and CT scan were the most performed exam respectively in 71% and 90%. PCE was localized in the head (31%), in the body (22%) and in the tail (47%). PCE compressed the common bile duct in 16 patients who had cyst in the head (26%). Cyst size was reported in 49 patients out of 62, the median diameter was 70 mm (IQR, 41–100 mm and range, 24–180 mm). Akbulut’s review (57) reported 54 patients with the following distributions: 22 in the head (41%), 6 in body and neck (11%), 26 in the tail or tail and body (48%). According to Akbulut’s review (57), PCE had a mean diameter of 71.3±36.1 mm (range, 26–180 mm). Safioleas et al. (47) found also that body and tail of the pancreas were the most common locations (4 out of 5).
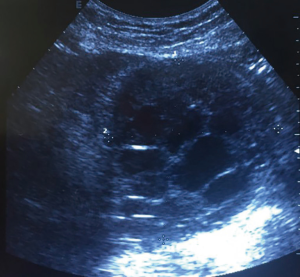
US is very useful and efficient to show calcification of the cystic wall, detachment of the membrane, and several daughter vesicles corresponding to rosette pattern (58). All these signs are pathognomonic of CE. Figure 2 shows a PCE with daughter vesicles corresponding to type III Gharbi classification (59) or CE2 of WHO-IWGE classification (60).
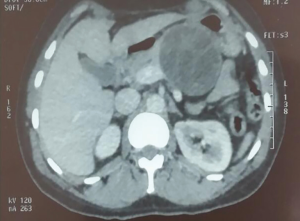
CT scan is efficient to detect early calcifications of the cystic wall. It is indicated when US is doubtful such as for type IV Gharbi classification which evokes a tumor, or when cyst has a big size and it is difficult to identify its localization with US (Figure 3).
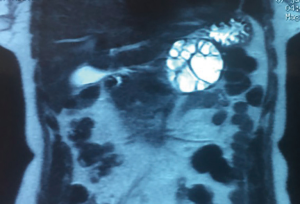
Magnetic resonance imaging (MRI): Figure 4 is a T2 MRI of the same patient who had a PCE of the tail (Figures 3,4). Zalaquett et al. (61) showed that the active cysts: CE1 has a very T2 hyper-intensive rim sign and CE2 has a T2 hyper-intensive daughter cysts which is pathognomonic. The transitional cyst CE3 has a T2 hypo-intense detached membrane. As concerns inactive cysts, CE4 has a T2 iso- to hypo-intense sign and CE5 has very hypo-intense sign.
Stojkovic et al. (62) compared retrospectively US, MRI and CT investigations of patients with CE to determine the performance of CT and MRI in comparison to the gold standard US. He concluded that ultrasound remains the cornerstone of diagnosis, staging and follow-up of CE cysts. MRI reproduces the ultrasound defined features of CE better than CT. If US cannot be performed due to cyst location or patient-specific reasons MRI with heavily T2-weighted series is preferable to CT (62).
Differential diagnosis
PCE associated to CE located in the liver is the frequent situation and diagnosis of PCE is easy to confirm. However, the diagnosis of isolated PCE, in spite of all these imaging features, sometimes hesitates with choledochal cyst (23,28,33), pseudocyst (18,26,30,32,39), cystic neoplasm of pancreas (31,38), and mucinous cystic neoplasm (56).
Treatment
Surgery is the cornerstone of PCE treatment. Surgical procedures should take into account location of the cyst, presence or not of a pancreatic ductal fistula communicating with the PCE.
The literature data did not help to decide. In this systematic review, Radical surgery was performed in 32 patients (52%) and conservative surgery in 30 cases (48%). Akbulut et al. in their review (57) reported 29 radical surgery out of 57 patients (51%) and 28 conservative surgery (25 by open approach + three percutaneous drainage) (49%). These data testimony that there is no consensus regarding therapeutic management.
Conclusions and recommendations
All levels of evidence are low and recommendations correspond to opinions of experts
What are the efficient tools to confirm the diagnosis of PCE?
Clinical presentation is not specific. Hydatid serology has a low sensitivity of 62%. Ultrasound remains the cornerstone of diagnosis, staging and follow-up of CE cysts. MRI reproduces the ultrasound defined features of CE better than CT. MRI with heavily T2-weighted series is preferable to CT. Pancreatic duct MRI should be promising to identify a fistula between PCE and pancreatic duct (level of evidence 3—recommendation B).
What is the best therapeutic strategy for the PCE?
Surgery is the main treatment of PCE. Open approach is validated, laparoscopic approach cannot be supported by a valid data. Furthermore, laparoscopic approach for the treatment of liver CE was associated with a higher rate of extrahepatic and peritoneal recurrence than open (63).
There are two surgical options: radical versus conservative surgery.
The decision depends on the location of PCE: Head versus body and/or tail of the pancreas. On other words, on the right or on the left of the mesenteric portal vein axis (64).
- Head of the pancreas: the tendency is toward conservative surgery (level of evidence 5—recommendation D);
- No fistula with pancreatic duct: unroofing procedure with omentoplasty;
- If there is a fistula between pancreatic duct and the PCE: anastomosis between PCE and digestive tract is indicated;
- Duodenopancreatectomy should be reserved exceptionally for selective patients.
- Body of the pancreas: the tendency is toward radical surgery (level of evidence 5—recommendation D);
- If presence of fistula between pancreatic duct and the cyst: central pancreatectomy or anastomosis between cyst and digestive tract;
- If no fistula: unroofing procedure with omentoplasty.
- Tail of the pancreas: the tendency is toward radical surgery (level of evidence 5—recommendation D).
- If presence of fistula between pancreatic duct and the cyst: distal pancreatectomy;
- If no fistula: distal pancreatectomy or unroofing procedure with omentoplasty.
PAIR is indicated for patients who does not accept surgery or when there is an anesthesia high risk. There is no available data.
Medical treatment (albendazole) should be prescribed 1 week before surgery and 2 months during postoperative period (level II evidence and grade C recommendation) (65).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dziri C. Hydatid disease A continuing surgical health problem World J Surg 2001;25:1-3. [Crossref] [PubMed]
- Chahed MK, Bellali H, Touinsi H, et al. Distribution of surgical hydatidosis in Tunisia, results of 2001-2005 study and trends between 1977 and 2005. Arch Inst Pasteur Tunis 2010;87:43-52. [PubMed]
- Kattan YB. Hydatid cysts in pancreas Br Med J 1975;4:729-30. [Crossref] [PubMed]
- Iacobiciu I, Stefănoiu V, Lazăr Z, et al. Aspects of hydatidosis in the adult population in Banat. Roum Arch Microbiol Immunol 1996;55:263-74. [PubMed]
- Dahniya MH, Hanna RM, Ashebu S, et al. The imaging appearances of hydatid disease at some unusual sites. Br J Radiol 2001;74:283-9. [Crossref] [PubMed]
- Vahedi MA, Vahedi ML. Demographics of patients with surgical and nonsurgical cystic echinococcosis in East Azerbaijan from 2001 to 2012. Pak J Biol Sci 2012;15:186-91. [Crossref] [PubMed]
- Bellil S, Limaiem F, Bellil K, et al. Descriptive epidemiology of extrapulmonary hydatid cysts: a report of 265 Tunisian cases Tunis Med 2009;87:123-6. [PubMed]
- Brunetti E, Kern P, Vuitton DA, et al. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010;114:1-16. [Crossref] [PubMed]
- Oxford Centre for Evidence-based Medicine – Levels of Evidence (March 2009). Available online: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
- Lada PE, Termengo D, Cáceres G, et al. Primary hydatid cyst of the pancreas. Rev Fac Cien Med Univ Nac Cordoba 2017;74:33-6. [PubMed]
- Agin M, Tumgor G, İcil S, et al. A rare cause of acute abdominal distention: opening of the pancreatic duct into hydatic cyst. Arch Argent Pediatr 2016;114:e346-8. [PubMed]
- Ahmed Z, Chhabra S, Massey A, et al. Primary hydatid cyst of pancreas: Case report and review of literature. Int J Surg Case Rep 2016;27:74-7. [Crossref] [PubMed]
- Mohamed H, Azza S, Chrif A, et al. Hydatid cyst of the pancreas revealed by acute pancreatitis: report of a case. Pan Afr Med J 2015;22:166. [PubMed]
- Eljai RS, Boufettal R, Farah RH, et al. Pancreatic hydatid cyst: a case about. Pan Afr Med J 2015;21:273. [PubMed]
- Lenz A, Brunner A, Metzger J. A Painless Mass in the Pancreas: Incidental Finding. JAMA Surg 2015;150:1189-90. [Crossref] [PubMed]
- Hiremath B, Subramaniam N, Boggavarapu M. Primary pancreatic hydatid cyst: an unexpected differential diagnosis. BMJ Case Rep 2015;2015:bcr2015211377. [Crossref] [PubMed]
- Mattous M, Belabbes S. Acute pancreatitis revealing a hydatid cyst of the pancreas. Pan Afr Med J 2015;20:429. [PubMed]
- Chinya A, Khanolkar A, Kumar J, et al. Isolated hydatid cyst of the pancreas masquerading as pancreatic pseudocyst. BMJ Case Rep 2015;2015:bcr2015211307. [Crossref] [PubMed]
- Kısaoğlu A, Özoğul B, Atamanalp SS, et al. Incidental isolated pancreatic hydatid cyst. Turkiye Parazitol Derg 2015;39:75-7. [Crossref] [PubMed]
- Sorogy ME, El-Hemaly M, Aboelenen A. Pancreatic body hydatid cyst: A case report. Int J Surg Case Rep 2015;6C:68-70. [Crossref] [PubMed]
- Yarlagadda P, Yenigalla BM, Penmethsa U, et al. Primary pancreatic echinococcosis. Trop Parasitol 2013;3:151-4. [Crossref] [PubMed]
- Trigui A, Rejab H, Guirat A, et al. Hydatid cyst of the pancreas. About 12 cases. Ann Ital Chir 2013;84:165-70. [PubMed]
- Mandelia A, Wahal A, Solanki S, et al. Pancreatic hydatid cyst masquerading as a choledochal cyst. J Pediatr Surg 2012;47:e41-4. [Crossref] [PubMed]
- Makni A, Jouini M, Kacem M, et al. Acute pancreatitis due to pancreatic hydatid cyst: a case report and review of the literature. World J Emerg Surg 2012;7:7. [Crossref] [PubMed]
- Karaman B, Battal B, Ustunsoz B, et al. Percutaneous treatment of a primary pancreatic hydatid cyst using a catheterization technique. Korean J Radiol 2012;13:232-6. [Crossref] [PubMed]
- Küçükkartallar T, Cakır M, Tekin A, et al. Primary pancreatic hydatid cyst resembling a pseudocyst. Turkiye Parazitol Derg 2011;35:214-6. [Crossref] [PubMed]
- Suryawanshi P, Khan AQ, Jatal S. Primary hydatid cyst of pancreas with acute pancreatitis. Int J Surg Case Rep 2011;2:122-4. [Crossref] [PubMed]
- Bhat NA, Rashid KA, Wani I, et al. Hydatid cyst of the pancreas mimicking choledochal cyst. Ann Saudi Med 2011;31:536-8. [Crossref] [PubMed]
- Masoodi MI, Nabi G, Kumar R, et al. Hydatid cyst of the pancreas: a case report and brief review. Turk J Gastroenterol 2011;22:430-2. [Crossref] [PubMed]
- Cankorkmaz L, Gümüş C, Celiksöz A, et al. Primary hydatid disease of the pancreas mimicking pancreatic pseudo-cyst in a child: case report and review of the literature. Turkiye Parazitol Derg 2011;35:50-2. [Crossref] [PubMed]
- Bansal VK, Misra MC, Krishna A, et al. Pancreatic hydatid cyst masquerading as cystic neoplasm of pancreas. Trop Gastroenterol 2010;31:335-7. [PubMed]
- Dalal U, Dalal AK, Singal R, et al. Primary hydatid cyst masquerading as pseudocyst of the pancreas with concomitant small gut obstruction--an unusual presentation. Kaohsiung J Med Sci 2011;27:32-5. [Crossref] [PubMed]
- Agrawal S, Parag P. Hydatid cyst of head of pancreas mimicking choledochal cyst. BMJ Case Rep 2011;2011:bcr0420114087. [Crossref] [PubMed]
- Varshney M, Shahid M, Maheshwari V, et al. Hydatid cyst in tail of pancreas. BMJ Case Rep 2011;2011:bcr0320114027. [Crossref] [PubMed]
- Shah OJ, Robbani I, Zargar SA, et al. Hydatid cyst of the pancreas. An experience with six cases. JOP 2010;11:575-81. [PubMed]
- Chammakhi-Jemli C, Mekaouer S, Miaoui A, et al. Hydatid cyst of the pancreas presenting with acute pancreatitis. J Radiol 2010;91:797-9. [Crossref] [PubMed]
- Karakas E, Tuna Y, Basar O, et al. Primary pancreatic hydatid disease associated with acute pancreatitis. Hepatobiliary Pancreat Dis Int 2010;9:441-2. [PubMed]
- Ibis C, Albayrak D, Altan A. Primary hydatid disease of pancreas mimicking cystic neoplasm. South Med J 2009;102:529-30. [Crossref] [PubMed]
- Bayat AM, Azhough R, Hashemzadeh S, et al. Hydatid cyst of pancreas presented as a pancreatic pseudocyst. Am J Gastroenterol 2009;104:1324-6. [Crossref] [PubMed]
- Pouget Y, Mucci S, O'Toole D, et al. Recurrent acute pancreatitis revealing a hydatid cyst of the pancreas. Rev Med Interne 2009;30:358-60. [Crossref] [PubMed]
- Bedioui H, Chebbi F, Ayadi S, et al. Primary hydatid cyst of the pancreas: Diagnosis and surgical procedures. Report of three cases. Gastroenterol Clin Biol 2008;32:102-6. [Crossref] [PubMed]
- Jai SR, El Hattabi K, Bensardi F, et al. Primary hydatid cyst of the pancreas causing obstructive jaundice. Saudi J Gastroenterol 2007;13:191-3. [Crossref] [PubMed]
- Moosavi SR, Kermany HK. Epigastric mass due to a hydatid cyst of the pancreas. A case report and review of the literature. JOP 2007;8:232-4. [PubMed]
- Faraj W, Selmo F, Khalifeh M, et al. Laparoscopic resection of pancreatic hydatid disease. Surgery 2006;139:438-41. [Crossref] [PubMed]
- Krige JE, Mirza K, Bornman PC, et al. Primary hydatid cysts of the pancreas. S Afr J Surg 2005;43:37-40. [PubMed]
- Ozmen MM, Moran M, Karakahya M, et al. Recurrent acute pancreatitis due to a hydatid cyst of the pancreatic head: a case report and review of the literature. JOP 2005;6:354-8. [PubMed]
- Safioleas MC, Moulakakis KG, Manti C, et al. Clinical considerations of primary hydatid disease of the pancreas. Pancreatology 2005;5:457-61. [Crossref] [PubMed]
- Echenique-Elizondo M, Amondarain Arratíbel JA. Hydatid disease of the pancreas. JOP 2004;5:51-2. [PubMed]
- Faucompret S, Farthouat P, Sainton T, et al. Complicated hydatid cyst of the pancreas after needle biopsy. Ann Chir 2001;126:491-2. [Crossref] [PubMed]
- Derbel F, Zidi MK, Mtimet A, et al. Hydatid cyst of the pancreas: a report on seven cases. Arab J Gastroenterol 2010;11:219-22. [Crossref]
- Lebon J, Bourgeon R, Claude R, et al. Hydatid cyst of the head of the pancreas with icterus and opening in Wirsung's canal; modern conception. Presse Med 1957;65:949-51. [PubMed]
- Morton PC, Terblanche JT, Bornman PC, et al. Obstructive jaundice caused by an intrapancreatic hydatid cyst. Br J Surg 1981;68:474-6. [Crossref] [PubMed]
- Mzali R, Ghorbal A, Shabou R, et al. Acute cholangitis caused by intrabiliary rupture of pancreatic hydatid cyst. Tunis Med 2004;82:470-4. [PubMed]
- Joske RA. Aetiological factors in the pancreatitis syndrome. Br Med J 1955;2:1477-81. [Crossref] [PubMed]
- Katkhouda N, Legoff D, Tricarico A, et al. Hydatid cyst of the pancreas responsible for chronic recurrent pancreatitis. Presse Med 1988;17:2021-3. [PubMed]
- Tezcaner T, Ekici Y, Aydın OH, et al. Laparoscopic spleen-preserving distal pancreatectomy for a primary hydatid cyst mimicking a mucinous cystic neoplasia. J Minim Access Surg 2017;13:148-50. [PubMed]
- Akbulut S, Yavuz R, Sogutcu N, et al. Hydatid cyst of the pancreas: report of undiagnosed case of pancreatic hydatid cyst and brief literature review. World J Gastrointest Surg 2014;6:190-200. [Crossref] [PubMed]
- Bouhaouala MH, Hendaoui L, Dziri C, et al. Abdominal hydatidosis – Pancreatic hydatid cyst (chapter 8). In: Bouhaouala MH, Hendaoui L. editors. Hydatid Disease: Imaging Features. 1st edition. Tunis, Tunisia: Research Medical Imaging Association 2008:107-10. ISBN 978-9973-0-0-358-4
- Gharbi HA, Hassine W, Brauner MW, et al. Ultrasound examination of the hydatic liver. Radiology 1981;139:459-63. [Crossref] [PubMed]
- WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop 2003;85:253-61. [Crossref] [PubMed]
- Zalaquett E, Menias C, Garrido F, et al. Imaging of Hydatid Disease with a Focus on Extrahepatic Involvement. Radiographics 2017;37:901-23. [Crossref] [PubMed]
- Stojkovic M, Rosenberger K, Kauczor HU, et al. Diagnosing and staging of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound? PLoS Negl Trop Dis 2012;6:e1880. [Crossref] [PubMed]
- Jerraya H, Khalfallah M, Osman SB, et al. Predictive factors of recurrence after surgical treatment for liver hydatid cyst. Surg Endosc 2015;29:86-93. [Crossref] [PubMed]
- Bouasker I, Zoghlami A, Ben Achour J, et al. Hydatid cysts of the pancreas, report of two cases. Tunis Med 2009;87:155-8. [PubMed]
- Dziri C, Haouet K, Fingerhut A. Treatment of hydatid cyst of the liver: where is the evidence? World J Surg 2004;28:731-6. [Crossref] [PubMed]
Cite this article as: Dziri C, Dougaz W, Bouasker I. Surgery of the pancreatic cystic echinococcosis: systematic review. Transl Gastroenterol Hepatol 2017;2:105.