Surgical treatment of gastrointestinal stromal tumors of the stomach: current status and future perspective
Introduction
The World Health Organisation (WHO) histological classification of gastric tumors are categorised into epithelial, non-epithelial and secondary tumors (1). Under non-epithelial gastric tumors, gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract (2). They are primarily located in the submucosa within muscularis propria or subserosa. GISTs are thought to originate from the pacemaker cells of the intestinal tract called interstitial cells of Cajal.
The discovery of gene mutation in KIT by Hirota et al., platelet-derived growth factor receptor alpha (PDGFRA) by Heinrich et al. and BRAF by Agaram et al. had led to the understanding of pro-growth signalling that drives GISTs (3-5). About 12–15% of adult GISTs and 90% of pediatric GISTs lacking KIT, PDGFRA or BRAF mutations are classified into succinate dehydrogenase (SDH)-deficient and non-SDH-deficient groups (6).
Complete surgical resection of the primary gastric GISTs remains the first line management. There are several surgical approaches and techniques described in the literature to achieve optimal surgical resection. Minimally invasive surgery is becoming more common and available in the curative intent resection of primary gastric GISTs. The increase in resectability and improvement in overall survival (OS) in the advanced, recurrent and metastatic GISTs treated with molecular targeted therapy in the form of tyrosine kinase inhibitor (TKI) is encouraging. Therefore, successful multimodal therapy of gastric GISTs requires adequate staging utilizing endoscopy, radiology, surgery, malignant potential risk assessment and mutational analysis in combination with molecular targeted therapy.
Demographic and clinical presentation of GISTs
The reported incidence of GISTs in most studies averages 1–2 cases per 100,000 people per year. The median age of GISTs diagnosis is 60–65 years and the male to female gender ratio is close to 1:1.
A systematic review of 15 studies totalling 2,456 patients with GISTs by Søreide et al. reported symptomatic disease in 81.3% (n=1,997) and incidental asymptomatic disease in 18.7% (7). Patients with GISTs commonly presented as abdominal pain in 61%, gastrointestinal bleeding such as hematemesis or melena in 58% and less commonly an intestinal obstruction or a palpable mass (8).
The anatomical locations of GISTs are frequently found in the stomach (55.6%), small bowel (31.8%), and are less frequently found in the colon and rectum (6%), other various locations (5.5%) and esophagus (0.7%) (7). Extra-gastrointestinal GISTs can be found in the mesentery, omentum and retroperitoneum (9).
An important epidemiological study by Coe et al. looking at the mortality rates of GISTs <2 cm using the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database identified significant increased 5-year GIST-specific mortality in those patients who had regional advanced GISTs (34%) or metastatic GISTs (34.3%) as compared to those with localized GISTs (5.6%) (10). It is therefore unwise to label the term ‘benign’ for any GISTs even with smaller sizes at the present time due to their adherent malignant potential risk.
Diagnosis and staging of gastric GISTs
The work up tests previously alluded in a review article by Lim et al. include an upper gastrointestinal endoscopy and a computed tomography (CT) scan of the thorax-abdomen-pelvis (11). Magnetic resonance imaging (MRI) scan and 18fluoro-deoxyglucose-positron emission tomography (18FDG-PET) scan may be required as part of staging tests due to other medical indications. Endoscopic ultrasound scan (EUS) may be useful in confirming the particular intestinal layers and depth of involvement of the GISTs before planning for surgery. It is possible to make an endoscopic and radiological diagnosis of GISTs based on the specific characteristics and appearances.
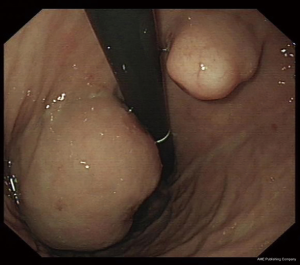
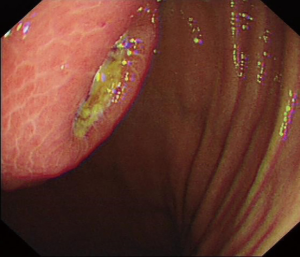
The typical endoscopic features of a GIST is a well-delineated and circumscribed spherical or hemispherical mass, arising mostly from submucosal muscle layer beneath the mucosa and pushing into the lumen to form a smooth-contoured elevation surrounded by a pseudocapsule (Figure 1). Focal mucosal ulceration (Figure 2) is commonly seen in symptomatic gastric GISTs. As gastric GISTs are mostly covered by normal mucosa, the conventional superficial tissue biopsies are often reported as negative histology for GISTs unless the biopsies are taken directly from the ulcer portion of the tumor or by using ‘bite-on-bite’ biopsy technique.
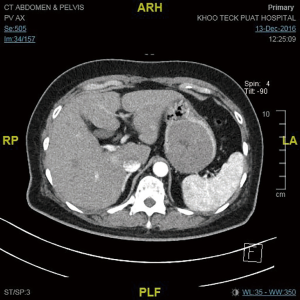
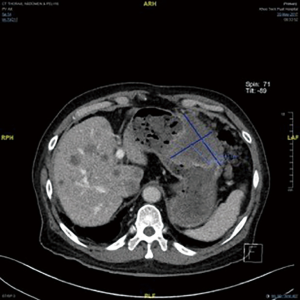
The CT scan features of GISTs vary depending on tumor size and organ of origin. Most GISTs arise within the muscularis propria have an exophytic growth pattern (Figure 3) and manifest as dominant masses outside the organ of origin. Dominant intramural and intraluminal masses are less common radiologic manifestations. GISTs occurring in the gastrointestinal tract and mesentery characteristically have hemorrhage, necrosis, or cyst formation that appear as focal areas of low attenuation on CT images (9). Metastatic gastric GISTs can be accurately staged by the CT scan (Figure 4).
In patients who have medical contraindications for CT scan study due to contrast allergy or chronic kidney disease may undergo staging MRI scan. The MRI scan features of gastric GISTs vary depending on the degree of necrosis and hemorrhage affecting the signal-intensity pattern. The solid portions of tumor are typically low signal intensity on T1-weighted images, are high signal intensity on T2-weighted images, and enhance after administration of gadolinium. The age of hemorrhage within the tumor vary from high to low signal intensity on both T1- and T2-weighted images (12).
18FDG-PET scan is useful to show the degree of metabolic activities of primary, recurrent and metastatic gastric GISTs. The combined CT and 18FDG-PET scans allow interpretation of the size, site, volume and metabolic activities of GISTs. The metabolic response seen on 18FDG-PET scans in patients treated with TKIs such as imatinib, have been shown to be closely related to clinical benefit. Conversely, the lack of metabolic response on 18FDG-PET scan indicates primary resistance to the treatment and may help identify patients who would benefit from second line therapy. Re-emergence of metabolic activity within tumor sites following a period of therapeutic response indicates secondary resistance to the drug (13).
Pre-operative fine needle aspiration (FNA) biopsy can be used to confirm cytological and/or histological diagnosis with the accuracy of 81.6% (14). The preoperative tissue diagnosis can be accepted as not an absolute requirement in those cases needing surgical resection due to symptomatic primary gastric submucosal tumors (SMTs) unless the diagnosis remains in doubt. However, a tissue biopsy is recommended in metastatic or unresectable disease and in those with borderline resectability planning neoadjuvant therapy with a view of down-staging or down-sizing very large gastric GISTs.
Surgical management of gastric GISTs
The indications for surgical treatment can be divided broadly into two groups: (I) curative intent primary gastric GISTs resection and (II) palliative intent advanced, recurrent or metastatic gastric GISTs resection.
Curative intent primary gastric GISTs resection
Surgical resection of the primary gastric GISTs with complete resection margin is the standard treatment. As GISTs rarely metastasize via lymphatic vessels, formal D1 or D2 lymphadenectomy is therefore not indicated unless there is a pathological enlarged locoregional lymph node. GISTs that are adherent or have invaded to surrounding organ or viscus would necessitate en bloc resection in order to achieve microscopic negative (R0) resection margin.
In a Japanese single centre clinicopathological review study of 140 patients with primary gastric GISTs treated between 1962 and 1999 showed different forms of gastric resections. The surgical approaches included 95 (68%) wedge resections, 21 distal gastrectomies, 18 proximal gastrectomies, 5 total gastrectomies and 1 enucleation (15). Sixty-two (44%) patients had lymph node dissection and all lymph nodes were negative for metastasis. This study identified male sex, tumor size of ≥10 cm and mitotic index >10 as the independent poor prognostic indicators.
The first laparoscopic Billroth II gastrectomy was reported in 1992 by Goh et al. for the management of gastric ulcer disease (16). Subsequently in 1994, Kitano et al. reported the first laparoscopic Billroth I gastrectomy for gastric cancer (17). Kitano had started to perform laparoscopic gastrectomy for early gastric cancer in December 1991 prior to those reports (18). Laparoscopic gastrectomy has since became widely practised and performed. In a Korean multicentre retrospective review of 406 consecutive patients who underwent curative resections for localised gastric GISTs between 1998 and 2012 showed that laparoscopic wedge resections (LWR) were performed successfully in 38.4% and open resections in 61.6% (19). There were 11 recurrent GIST cases (2.7%) in open resection group and none in LWR group. The mean tumour size was 3.45 cm in the LWR group versus 5.46 cm in the open group. This study confirmed LWR of primary gastric GISTs is feasible.
In an American case series of 155 primary gastric GISTs resections performed over a 12-year period between 1998 and 2009 were identified for analysis (20). Forty cases of consecutive laparoscopic resection were matched by tumor size to patients with open resection. The study revealed laparoscopic resection of GISTs ≤8 cm resulted in a shorter hospital stays with similar oncological outcomes compared to an open resection with a median follow-up of 34 months. There were 13 conversions to open surgery, 5 of these were secondary to tumor location at the gastro-esophageal junction or lesser curve.
In a Chinese cohort of 214 patients with primary gastric GISTs treated between 2006 and 2014, a comparative study of GISTs located at unfavorable sites (n=74 cases) versus favorable sites (n=140 cases) were analyzed (21). The unfavorable sites were the gastro-esophageal junction, lesser curve, posterior wall, antrum and pylorus. The favorable sites were the gastric fundus, anterior wall, and greater curve. Both open (n=81) and laparoscopic (n=133) resections were carried out in the two groups. The study showed the wedge resection rate mostly performed laparoscopically was higher in the favorable group than the unfavorable group. Laparoscopic resections in both groups resulted in a shorter operative time, lower blood loss, shorter time to first flatus and to first fluid diet, and shorter postoperative stay than open resections. The mean tumor size was 5.3 cm in the favorable group versus 4.8 cm in the unfavorable group. The mean tumor size in the laparoscopic group was generally about 1 cm less than the open group. There were no differences in the 5-year OS and recurrence-free survival (RFS) of these groups regardless of open or laparoscopic resection.
In another Chinese cohort study of 266 patients with gastric SMTs treated from 2006 and 2016 were analyzed. Gastric GISTs were diagnosed histologically in 229 patients. Out of the 229 patients, 203 patients underwent laparoscopic exogastric wedge resection (LEWR) whilst the remaining 26 patients with tumors near esophagogastric junction or antrum underwent laparoscopic transgastric resection (LTR) (22). The concern of stenosis or deformity at the gastric inlet or outlet created by LEWR that may require extensive total, proximal or distal gastrectomy was addressed by LTR technique. The mean tumor size was 3.6 cm in the LEWR group versus 2.1 cm in the LTR group. The study concluded both LEWR and LTR were successfully performed without mortality. The low complication rate of 4.4% was related to intraluminal bleeding, delayed gastric emptying and pneumonia.
A new technique called laparoscopic endoscopic cooperative surgery (LECS) was described to treat gastric GISTs in a small Japanese case series (23). LECS was shown to be safe and feasible for smaller gastric GISTs less than 5 cm with the outcomes similar to conventional LWR. The advantage of LECS is the reduction in the resected area of the gastric wall compared to conventional LWR using a linear stapler. LECS is purported to be an alternative to a difficult or failed endoscopic submucosal dissection (ESD) for gastric tumor that fits the criteria for endoscopic resection.
In another Japanese study, single incision laparoscopic surgery (SILS) partial gastrectomy was attempted in 12 consecutive patients with gastric SMTs in a single institution (24). These gastric SMTs were located in the greater curve or anterior wall of the stomach and the median tumor size was 3 cm. The lesions were mobilised and resected with endoscopic stapling device through the umbilicus using SILS technique successfully without any additional trocars.
The current evidence shows that gastric GISTs resection performed conventionally through open surgery can now be achieved frequently by minimal invasive surgery with equivalent safety efficacy. The decision to perform an open or a laparoscopic surgery depends on the site, size and local invasion of the primary gastric GISTs. Laparoscopic gastric GISTs surgery has many advantages and more importantly it can achieve similar oncological outcomes compared to open surgery. LWR (Figure 5) is the preferred choice for most GISTs, although partial gastrectomy (Figure 6) or total gastrectomy may be necessary in some complex cases. During the surgical dissection and resection, care must be taken to avoid disrupting the pseudocapsule of the tumour and more importantly intraperitoneal implantation. Although complete surgical R0 resection of gastric GISTs may represent curative treatment, but certain high risk features of the resected GISTs can still give rise to recurrence of the disease.
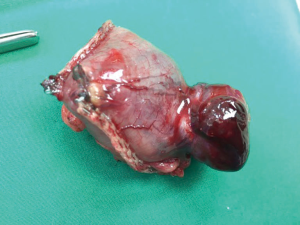
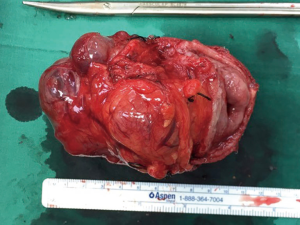
Palliative intent advanced, recurrent and metastatic gastric GISTs resection
The response to traditional chemotherapeutic agents and radiotherapy for the treatment of gastric GISTs has been dismal and therefore not recommended (25). The treatment outcomes of metastatic GISTs using hepatic artery embolization and debulking surgery followed by intraperitoneal chemotherapy have been investigated and have discouraging results (26,27). Although GISTs are considered radiation-resistant, palliative radiotherapy may benefit those with bone and soft tissue GISTs metastases through symptomatic relief and stabilization of target lesions (28).
The discovery of imatinib has revolutionized the treatment of GISTs. Many clinical trials have shown the benefit of imatinib in advanced, unresectable and metastatic GISTs (29-32). Imatinib was approved by the United States Food and Drug Administration for the treatment of unresectable or metastatic GISTs in 2002 and for adjuvant use in high-risk resected GISTs patients to prevent recurrence in 2008. Patients with advanced, unresectable or metastatic gastric GISTs who were treated with imatinib may undergo palliative intent surgical resection at a later date. Imatinib may reduce the tumor volume and render the primary disease resectable. Surgery for residual disease has been suggested for non-refractory metastatic GISTs to reduce the likelihood of resistant to imatinib from secondary mutation. Re-excision of an inadvertent incomplete resection margin and those with recurrent GISTs should be considered on individual basis.
Many studies have shown that surgical resection of residual, advanced and metastatic GISTs disease after treatment with imatinib have better outcomes (33-36). At 2 years, the OS was 100% in those who responded to imatinib followed by surgical resection compared to the OS of 36–60% in those with progressive disease (33,34).
In a long term follow up of two European studies of over 170 patients in each study who underwent complete resection metastasectomy after treatment with imatinib showed a median OS of 7.3–8.7 years in patients with complete remission (37,38). However, incomplete resection and debulking surgery does not prolong survival compared to treatment with imatinib alone. Similarly there is little or no benefit of surgery in the setting of generalized progression with metastatic GIST or multifocal resistance while on imatinib (39). These patients should be considered for clinical trials of new systemic agents. These new systemic agents were previously discussed in the review article by Lim et al. (11).
Gastric GISTs trial studies
There are many newer pharmacotherapy-related GISTs studies being carried out which can be found on www.clinicaltrials.gov website. There are only a few surgical intervention-related gastric GISTs studies as summarized in Table 1. These trials will address some of the future perspective of surgical management of gastric GISTs such as the role of robotic surgery and endoscopic resection. Robotic surgery has been increasing performed in the last decade but the current evidence showed that robotic gastric resections have the disadvantages of longer operating time and higher costs than conventional laparoscopic approach (40).
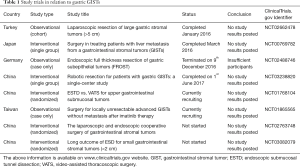
Full table
Endoscopic enucleation of gastric GISTs has been described which includes the techniques of ESD and endoscopic submucosal tunnel dissection (ESTD) (41,42). Endoscopic enucleation may have some advantages such as keeping the stomach intact, short hospital stay, a conscious sedation procedure, relatively low cost and fewer manpower required compared to surgery. However, there are concerns of incomplete resection, the risk of perforation, spillage and seedling. Currently endoscopic enucleation is not frequently performed and LECS may appear to be the safer alternative.
Malignant potential risk assessment of resected gastric GISTs
Historical assessment of the malignant potential in GISTs were based on the criteria of tumor size, mitotic count, proliferating cell nuclear antigen and proliferation index, which allowed classification into low and high-risk subgroups (43). Subsequent risk stratification systems for GISTs were proposed, such as the National Institutes of Health (NIH) consensus criteria based on size and mitotic count (also known as Fletcher’s criteria) and the Armed Forces Institute of Pathology (AFIP) criteria based on size, mitotic count and tumor site (also known as Miettinen’s criteria) (44,45).
The NIH risk criteria based on GISTs at all sites was later revised into gastric and non-gastric GISTs (also known as Joensuu’s criteria) (46). This is to recognise gastric GISTs have a lower risk of recurrence than non-gastric tumors of the same size and mitotic count. According to the NIH criteria for primary GISTs, the distribution of risk is categorized as very low risk (15%), low risk (30%), intermediate risk (22%) and high risk (33%).
The 7th edition of the International Union Against Cancer (UICC) utilizing TNM classification in addition to a grade category based on mitotic count was introduced for GISTs and was later updated in the 8th edition (47,48). Table 2 shows the commonly used criteria for assessing malignant potential risk of gastric GISTs. Other factors associated with a higher malignant risk of GISTs not included in those criteria are the presence of tumor necrosis, invasion to serosa or adjacent structure, rich vascularity, incomplete resection margin, tumor rupture and spillage during surgery (49).

Full table
One important point to note is the variation of reported microscopic positive (R1) resection margin. North American guidelines define R1 as the presence of tumor cells at the surface of the resection margin (0 mm) whereas the British Royal College of Pathology guidelines define R1 as the presence of tumor cells within 1 mm of the resection margin. The lack of international consensus for the definition of margin involvement explains the high variation in the reported R1 rates.
By categorizing the malignant risk potential of the resected gastric GISTs, clinicians can counsel and advise the patients and follow the current treatment guidelines. A proposed algorithm for the management of GISTs based on the current guidelines can be found in the review article by Lim et al. (11).
Follow up and prognosis of resected gastric GISTs
In a large series of 200 patients with GISTs treated and followed-up at a single institution from 1982–1998 predated the use of TKIs, found that 46% had primary disease, 47% had metastasis and 7% had isolated local recurrence (50). Eighty patients with primary disease who underwent complete resection had 5-year survival rate of 54%. Survival was predicted by tumor size and tumor recurrence was noted to occur at the original primary tumor site, peritoneum and liver.
The American College of Surgeons Oncology Group led a trial studying the long-term outcome of 106 patients categorized as high risk of recurrence who underwent complete gross GISTs resection followed by adjuvant imatinib at 400 mg/d for 1 year from 2001 to 2003 (51). After a median follow-up of 7.7 years, the 1-, 3- and 5-year OS rates were 99%, 97% and 83% respectively. The 1-, 3- and 5-year RFS rates were 96%, 60% and 40% respectively. The lower OS rates were associated with older age and high mitotic rate whilst the lower RFS rates were associated with larger tumor size, KIT exon 9 mutation, high mitotic rate and older age.
In a multicentre observational study from Korea and Japan, the long term outcome of 1,057 gastric GISTs patients who underwent surgery between 2000 and 2007 was analyzed (52). It is important to note only 108 patients received imatinib in the study due to limitation of national health insurance. According to the TNM system, the 5-year RFS rates were 95–99% in stage I, 94.1% in stage II, 74.1% in stage IIIA, and 48.6% in stage IIIB patients. According to the modified NIH classification, the 5-year RFS rates were 98–99% in very low- or low-risk patients, 96.3% in intermediate-risk patients, and 74.9% in high-risk patients. In the subgroup analysis of high risk patients according to TNM system, the rates were 91.6% in stage II, 74.1% in stage IIIA, and 48.6% in stage IIIB patients. On multivariate analysis, the independent factors for gastric GISTs recurrence following surgery were gender, tumor size, mitotic count, and radicality of resection (R0–R2). The treatment outcome and prognosis of gastric GISTs in Korea and Japan even with low imatinib uptake seems more favorable compared to those in Western countries. The study concluded the 7th UICC TNM system is more reflective of the 5-year RFS of patients with gastric GIST when compared to the modified NIH risk classification.
A very large cohort of 5,139 patients with resected and metastatic GISTs were analysed using the data extracted from SEER database from 1998 to 2011 (53). GISTs were located in the stomach in 58.7% and in small bowel in 31.2%. Lymph node and distant metastases were found in 5.1% and 18.0% respectively. For non-metastatic GISTs, 3-year OS increased from 68.5% in 1998 to 88.6% in 2008 whilst the cancer-specific survival (CSS) improved from 75.3% to 92.2% in the same period. For metastatic GISTs, 3-year OS increased from 15.0% in 1998 to 54.7% in 2008 whilst the CSS improved from 15.0% to 61.9% in the same period. This study identified larger tumor size, location other than stomach or small bowel, nodal or distant metastases, older age, earlier time point of diagnosis, male gender and single marital status are associated with significantly worse OS and CSS.
Conclusions
Primary gastric GISTs resection can be performed frequently by minimal invasive surgery with similar oncological outcomes. Whilst patient factors (older age and male) and tumor factors (size and mitotic index) may predict the prognostication, it is imperative that surgeons focus on the surgical factors (incomplete resection margin, tumor rupture or spillage) when selecting the type of surgical resection techniques. All these factors influence the final oncological outcome, RFS and OS. Intermediate and high risk groups of resected primary gastric GISTs according to the current risk stratification criteria should be considered for mutational analysis and molecular targeted therapy where treatment are available and affordable as part of the personalised multimodal therapy. Patients with unresectable or metastatic gastric GISTs if responded to targeted therapy may benefit from metastasectomy. It is very encouraging evidence to see the OS and CSS has improved in the last 2 decades, not only in non-metastatic but also in metastatic GISTs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Hamilton SR, Aaltonen LA. World Health Organization classification of tumors: pathology and genetics of tumours of the digestive system. Lyon, France: IARC, 2000.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001;438:1-12. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708-10. [Crossref] [PubMed]
- Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer 2008;47:853-9. [Crossref] [PubMed]
- Gaal J, Stratakis CA, Carney JA, et al. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol 2011;24:147-51. [Crossref] [PubMed]
- Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39-46. [Crossref] [PubMed]
- Caterino S, Lorenzon L, Petrucciani N, et al. Gastrointestinal stromal tumors: correlation between symptoms at presentation, tumor location and prognostic factors in 47 consecutive patients. World J Surg Oncol 2011;9:13. [Crossref] [PubMed]
- Levy AD, Remotti HE, Thompson WM, et al. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics 2003;23:283-304, 456; quiz 532.
- Coe TM, Fero KE, Fanta PT, et al. Population-Based Epidemiology and Mortality of Small Malignant Gastrointestinal Stromal Tumors in the USA. J Gastrointest Surg 2016;20:1132-40. [Crossref] [PubMed]
- Lim KT, Tan KY. Current research and treatment for gastrointestinal stromal tumors. World J Gastroenterol 2017;23:4856-66. [Crossref] [PubMed]
- Hasegawa S, Semelka RC, Noone TC, et al. Gastric stromal sarcomas: correlation of MR imaging and histopathologic findings in nine patients. Radiology 1998;208:591-5. [Crossref] [PubMed]
- Van den Abbeele AD. The lessons of GIST--PET and PET/CT: a new paradigm for imaging. Oncologist 2008;13 Suppl 2:8-13. [Crossref] [PubMed]
- Yoshida S, Yamashita K, Yokozawa M, et al. Diagnostic findings of ultrasound-guided fine-needle aspiration cytology for gastrointestinal stromal tumors: proposal of a combined cytology with newly defined features and histology diagnosis. Pathol Int 2009;59:712-9. [Crossref] [PubMed]
- Fujimoto Y, Nakanishi Y, Yoshimura K, et al. Clinicopathologic study of primary malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer 2003;6:39-48. [Crossref] [PubMed]
- Goh P, Tekant Y, Kum CK, et al. Totally intra-abdominal laparoscopic Billroth II gastrectomy. Surg Endosc 1992;6:160. [Crossref] [PubMed]
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-8. Erratum in: Surg Laparosc Endosc 2013;23:480. [PubMed]
- Kitano S, Ohta M, Shiraishi N. Gastric resection for advanced gastric cancer. In: Assalia A, Gagner M, Schein M. editors. Controversies in Laparoscopic Surgery. Springer-Verlag Berlin Heidelberg, 2006:185-92.
- Kim IH, Kim IH, Kwak SG, et al. Gastrointestinal stromal tumors (GISTs) of the stomach: a multicenter, retrospective study of curatively resected gastric GISTs. Ann Surg Treat Res 2014;87:298-303. [Crossref] [PubMed]
- Karakousis GC, Singer S, Zheng J, et al. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol 2011;18:1599-605. [Crossref] [PubMed]
- Huang CM, Chen QF, Lin JX, et al. Can laparoscopic surgery be applied in gastric gastrointestinal stromal tumors located in unfavorable sites?: A study based on the NCCN guidelines. Medicine (Baltimore) 2017;96:e6535. [Crossref] [PubMed]
- Chen K, Pan Y, Zhai ST, et al. Short-term outcomes of laparoscopic local resection for gastric submucosal tumors: a single-center experience of 266 patients. BMC Surg 2017;17:33. [Crossref] [PubMed]
- Namikawa T, Hanazaki K. Laparoscopic endoscopic cooperative surgery as a minimally invasive treatment for gastric submucosal tumor. World J Gastrointest Endosc 2015;7:1150-6. [Crossref] [PubMed]
- Takata A, Nakajima K, Kurokawa Y, et al. Single-incision laparoscopic partial gastrectomy for gastric submucosal tumors without compromising transumbilical stapling. Asian J Endosc Surg 2014;7:25-30. [Crossref] [PubMed]
- Dematteo RP, Heinrich MC, El-Rifai WM, et al. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol 2002;33:466-77. [Crossref] [PubMed]
- D'Amato G, Steinert DM, McAuliffe JC, et al. Update on the biology and therapy of gastrointestinal stromal tumors. Cancer Control 2005;12:44-56. [Crossref] [PubMed]
- Maluccio MA, Covey AM, Schubert J, et al. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer 2006;107:1617-23. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Collan J, et al. Radiotherapy for GIST progressing during or after tyrosine kinase inhibitor therapy: A prospective study. Radiother Oncol 2015;116:233-8. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [Crossref] [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [Crossref] [PubMed]
- Chaudhry UI, DeMatteo RP. Management of resectable gastrointestinal stromal tumor. Hematol Oncol Clin North Am 2009;23:79-96. viii. [Crossref] [PubMed]
- Gronchi A, Fiore M, Miselli F, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 2007;245:341-6. [Crossref] [PubMed]
- DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg 2007;245:347-52. [Crossref] [PubMed]
- Andtbacka RH, Ng CS, Scaife CL, et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol 2007;14:14-24. [Crossref] [PubMed]
- Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006;24:2325-31. [Crossref] [PubMed]
- Rubió-Casadevall J, Martinez-Trufero J, Garcia-Albeniz X, et al. Role of surgery in patients with recurrent, metastatic, or unresectable locally advanced gastrointestinal stromal tumors sensitive to imatinib: a retrospective analysis of the Spanish Group for Research on Sarcoma (GEIS). Ann Surg Oncol 2015;22:2948-57. [Crossref] [PubMed]
- Bauer S, Rutkowski P, Hohenberger P, et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib -- analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol 2014;40:412-9. [Crossref] [PubMed]
- Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated With Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Nakauchi M, Uyama I, Suda K, et al. Robotic surgery for the upper gastrointestinal tract: Current status and future perspectives. Asian J Endosc Surg 2017;10:354-63. [Crossref] [PubMed]
- Inoue H, Ikeda H, Hosoya T, et al. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy 2012;44:225-30. [Crossref] [PubMed]
- Ye LP, Zhang Y, Mao XL, et al. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 2014;28:524-30. [Crossref] [PubMed]
- Franquemont DW. Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol 1995;103:41-7. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Sobin LH, Gospodarowics MK, Wittekind C. TNM classification of malignant tumours. International union against cancer (UICC). 7th edition. New York: Wiley, 2010:78-81.
- Brierley JD, Gospodarowicz, Wittekind C. TNM classification of malignant tumours. International union against cancer (UICC). 8th edition. New York: Wiley-Blackwell, 2016;127-30.
- Gronchi A. Risk stratification models and mutational analysis: keys to optimising adjuvant therapy in patients with gastrointestinal stromal tumour. Eur J Cancer 2013;49:884-92. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg 2013;258:422-9. [Crossref] [PubMed]
- Kim MC, Yook JH, Yang HK, et al. Long-Term Surgical Outcome of 1057 Gastric GISTs According to 7th UICC/AJCC TNM System: Multicenter Observational Study From Korea and Japan. Medicine (Baltimore) 2015;94:e1526. [Crossref] [PubMed]
- Güller U, Tarantino I, Cerny T, et al. Population-based SEER trend analysis of overall and cancer-specific survival in 5138 patients with gastrointestinal stromal tumor. BMC Cancer 2015;15:557. [Crossref] [PubMed]
Cite this article as: Lim KT. Surgical treatment of gastrointestinal stromal tumors of the stomach: current status and future perspective. Transl Gastroenterol Hepatol 2017;2:104.


