Survival impact of the number of lymph node retrieved on patients with node-negative gastric cancer: more is better?
Adequate and proper lymphadenectomy has been widely accepted as a standard treatment for localized gastric cancer (GC) (1-3). The number of lymph node involvement is one of the parts of TNM classification based on American Joint Committee on Cancer (4). Accordingly, accuracy of nodal metastasis is important to predict patient’s survival precisely. In the issue of Annals of Surgical Oncology, Deng et al. investigated whether increasing the number of examined lymph node (NELN) will affect prognosis of node-negative GC patients after radical resection (5). Their results indicated that increasing the NELN improves the accuracy of cancer staging, especially in advanced stage disease. Furthermore, the authors also concluded that based on the discrepancy findings between China and Japan, inexperienced pathologists in China may potentially result in the low NELN, which led to incorrect cancer staging (5). Besides, they also observed that Chinese patients had more advanced diseases and less mean NELN as compared with Japanese patients. These are the principal causes explaining worse overall survival (OS) in Chinese patients than Japanese patients with regard to NELN.
Quality control or measurement of GC surgery has been emphasized the importance of the NELN. A study reported by Morgan et al. using Surveillance, Epidemiology, and End Results Cancer Registry of Greater California and California Cancer Registry including 3,321 stage I to III GC patients undergoing surgery found that 42.3% of patients had ≥15 lymph nodes harvested and survival was improved in Cox proportional hazards regression with 0.70 of hazard ratio [95% confidence interval (CI), 0.63–0.78] (6). Another research including two major high-volume medical centers in Taiwan with recruitment of 5,386 GC patients who received curative surgery suggested that retrieving >25 lymph nodes substantially improve patients’ survival without compromising patient safety (7). Nonetheless, the survival impact of NELN on the subgroup of node-negative GC patients cannot be elucidated since these studies enrolled node-positive and node-negative patients. In this regard, the impact of NELN on prognosis in node-negative GC patients has been investigated in several retrospective studies (5,8-11). Deng et al. identified the optimal cut-off value of NELN as 15 and 35 in predicting patients’ outcomes, suggesting that NELN >35 should be considered essential for T4N0 cases to evaluate the accuracy of pathological stage (5). Ji et al. indicated that patients with NELN ≥22 had better 5-year survival as compared with those with NELM <22, especially for those at T4 status (10). According to the Bayesian model proposed by Martinez-Ramos et al., the NELN ≤9 was viewed to have a high risk (HR) of misclassification; 10–25 and ≥26 were considered as a moderate risk (MR) and low risk (LR), respectively. A significant improvement in the disease-specific survival was observed for the MR pN0 and LR pN0 but not for the HR pN0 patients as compared to pN1 patients (11). Our previous studies have showed that advanced GC patients with NELN >25 had better outcomes than those with NELN ≤25 (8).
Deng et al. found that the survival difference was not evident between patients with T1N0 and T1N1 (the number of nodal involvement ≤2) when NELN was >15 (5). These results supported the current treatment plans for stage I (T1N0, T1N1 or T2N0) GC patients who underwent curative surgery that no adjuvant therapies are recommended. Interestingly, their findings also indicated that survival benefits did not differ between patients with T2N1 and T2N0 stratified by NELN (5). However, whether patients with T2N1 received adjuvant chemotherapy or not was not mentioned in their study (5). Therefore, no survival difference between patients with T2N1 and T2N0 should be interpreted with caution since studies have revealed that adjuvant chemotherapy improved OS in stage II–III GC after D2 dissection (12,13). The impact of limited nodal metastasis or adjuvant chemotherapy on survival cannot be evaluated without the information of chemotherapy for T2N1 lesion (5).
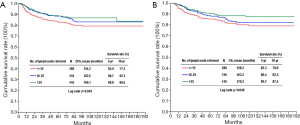
Deng et al. explored the prognostic factors for survival in a large-scale study enrolling 1,137 node-negative GC patients (5). In line with their observation, our study (n=1,030) indicated that tumor size, tumor location, NELN, and T4 lesion were independent prognostic factors for disease-specific OS (8). Moreover, our study also showed that the presence of perineural invasion was an unfavorable predictor for outcome on multivariate analysis (8). However, this important parameter was not included into survival analysis in Deng’s report (5). Table 1 summarizes the survival impact of NELN on patients with node-negative GC from relevant investigations. Although there were some differences in percentages of patients in terms of T status or extent of lymphadenectomy among each study, the results consistently indicated that advanced T stage and NELN were independent predictors for OS. The 5-year survival rate was 53% reported by Jin et al. (14), significantly lower than that of 69.8% and 88.5% from Deng et al. (5) and Hsu et al. (8), respectively. The crucial reason to explain different survival figures among the 3 series is the NELN. The mean and median nodes examined were only 17 and 16, respectively in the Jin’s study (14), much less than those in the other 2 researches (5,8). It should be also noted that in the large-scale cohort studies such as Deng’s and ours, 5-year survival rates were >90% in patients with NELN >35 or 25 (5,8). Figure 1 depicts our cumulative disease-free survival (DFS) and disease-specific OS rates of patients with node-negative T1–T4 GC (n=1,030) according to the NELN. The 5-year disease-specific survival rates were 85.3%, 88.4% and 90.7% in NELN <15, 16–25 and >25, respectively (P=0.048).

Full table
Our previous results indicated that T1 lesion had 96.3% of 5-year survival rates after radical resection (8), similar to the Deng’s value in Japanese patients (5). In the investigation from Chou et al., the mean NELN did not differ between patients with T2–T4 lesion (26 vs. 24) in terms of recurrence after median 78.7-month follow-up, suggestive of other factors which might affect tumor recurrence (15). Relapse was identified in 18.9% of patients (n=85) in the whole cohort, which did not include T1 lesion. However, Deng et al. including 466 patients with T1 and 671 with T2–4 tumors found that a total of 400 patients (35.2%) died from disease (5). Comparing with our previous data (15), higher recurrence rates in Deng’s series might be partially explained by the fact that among 671 patients with T2–4 tumor (59%), only 20% of them underwent ≥D2 lymphadenectomy or among 687 patients, 60.4% of them had NELN >15 (5). Furthermore, Deng et al. also identified that Japanese patients had considerably more NELN than Chinese patients, and the NELN was noted to be an independent prognostic factor for Chinese patients rather than Japanese patients, suggesting that inadequate lymphadenectomy or insufficient NELN may occur in the Chinese group.
The possible reason why increasing NELN can improve outcomes in node-negative patients may be associated with reducing stage migration by accurate pathological staging and removing occult tumor cells such as micrometastasis or isolated tumor cells. In this regards, a systemic review and meta-analysis of 1,478 patients revealed that occult lymph node metastasis conferred unfavorable disease-specific OS. The subgroup analysis showed that nodal micrometastasis had worse survival in patients with early GC but not for those who underwent D2 lymphadenectomy (16). In addition, another meta-analysis enrolling 18 studies and 1,569 patients demonstrated that lymph node micrometastasis was correlated with worse 5-year survival (17).
Treatment strategies for localized resectable node-negative GC comprise D1 or D2 lymph node dissection depending on the depth of tumor invasion and status of nodal involvement based on the Japanese GC treatment guidelines published in 2017 (18). D1 or D1+ lymphadenectomy is recommended in treating clinical T1a or T1b tumor without lymph node metastasis, whereas D2 is suggested for ≥T2 lesion or an index of suspicion to nodal metastasis (18). Our previous research enrolling 1,030 GC patients observed that 5-year survival rates did not differ in node-negative T1 patients with NELN ≤15 or >15; however, T2–T4 patients with NELN >25 had favorable survival (8). Notably, the vast majority of patients was operated on by 10 experienced gastric surgeons, who had each contributed >100 cases in our study (8). Similar to our findings, Deng’s report showed that the survival difference was not evident in Japanese patients (including 79% early cases) with NELN ≤15 as compared to those with 16–25, whereas the survival difference was statistically significant in Chinese patients (including 93% advanced cases) in terms of the NELN ≤15, 16–23 and >35 (5). These findings collectively suggested that the more lymph nodes are harvested, the more survival benefits gain in node-negative advanced GC.
Theoretically, NELN, to some extent, is correlated with extent of lymphadenectomy and is more practical in evaluating the quality of GC surgery. The NELN is determined by either the extent of lymphadenectomy such as <D2 and ≥D2 by the surgeon or the retrieval from the surgical specimen by the surgeon or the pathologist. The diligence and attitude to deal with the surgical sample will greatly influence the NELN. Standardization of GC surgery and managing the surgical specimen is mandatory to maintain the quality of care for GC since inadequate lymphadenectomy and underestimation of the nodal status will lead to insufficient treatment and incorrect staging. In case of NELN <25 is reported while D2 dissection was claimed by the experienced surgeon, it is mandatory to re-examine the surgical specimen with an attempt to increase NELN and therefore to classify cancer stage correctly.
We propose that adjunct therapies including chemotherapy or radiation may benefit advanced node-negative GC patients with NELN <25 or negative prognostic factors such as tumor size >3.5 cm, T4 lesion and presence of perineural invasion to reduce recurrence rates and prolong patients’ survival (19). Nonetheless, a prospective randomized controlled trial is needed for validating the treatment strategy for those patients with poor prognostic factors, and useful biomarkers are urgently necessary for predicting efficacy of adjuvant treatment in the era of precision medicine.
Acknowledgements
Funding: This work was partly supported by the Chang Gung Medical Research Program, Taiwan (CORPG3E0152 and CORPG3E0153).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mocellin S, McCulloch P, Kazi H, et al. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev 2015.CD001964. [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Hsu JT, Liu MS, Wang F, et al. Standard radical gastrectomy in octogenarians and nonagenarians with gastric cancer: are short-term surgical results and long-term survival substantial? J Gastrointest Surg 2012;16:728-37. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer. 7th edition. New York: Springer, 2009.
- Deng J, Yamashita H, Seto Y, et al. Increasing the Number of Examined Lymph Nodes is a Prerequisite for Improvement in the Accurate Evaluation of Overall Survival of Node-Negative Gastric Cancer Patients. Ann Surg Oncol 2017;24:745-53. [Crossref] [PubMed]
- Morgan JW, Ji L, Friedman G, et al. The role of the cancer center when using lymph node count as a quality measure for gastric cancer surgery. JAMA Surg 2015;150:37-43. [Crossref] [PubMed]
- Liu YY, Fang WL, Wang F, et al. Does a Higher Cutoff Value of Lymph Node Retrieval Substantially Improve Survival in Patients With Advanced Gastric Cancer?-Time to Embrace a New Digit. Oncologist 2017;22:97-106. [Crossref] [PubMed]
- Hsu JT, Lin CJ, Sung CM, et al. Prognostic significance of the number of examined lymph nodes in node-negative gastric adenocarcinoma. Eur J Surg Oncol 2013;39:1287-93. [Crossref] [PubMed]
- Jiao XG, Deng JY, Zhang RP, et al. Prognostic value of number of examined lymph nodes in patients with node-negative gastric cancer. World J Gastroenterol 2014;20:3640-8. [Crossref] [PubMed]
- Ji X, Bu ZD, Li ZY, et al. Prognostic significance of the total number of harvested lymph nodes for lymph node-negative gastric cancer patients. BMC Cancer 2017;17:558. [Crossref] [PubMed]
- Martinez-Ramos D, Calero A, Escrig-Sos J, et al. Prognosis for gastric carcinomas with an insufficient number of examined negative lymph nodes. Eur J Surg Oncol 2014;40:358-65. [Crossref] [PubMed]
- Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389-96. [Crossref] [PubMed]
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [Crossref] [PubMed]
- Jin LX, Moses LE, Squires MH 3rd, et al. Factors Associated With Recurrence and Survival in Lymph Node-negative Gastric Adenocarcinoma: A 7-Institution Study of the US Gastric Cancer Collaborative. Ann Surg 2015;262:999-1005. [Crossref] [PubMed]
- Chou HH, Kuo CJ, Hsu JT, et al. Clinicopathologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am J Surg 2013;205:623-30. [Crossref] [PubMed]
- Huang JY, Xu YY, Li M, et al. The prognostic impact of occult lymph node metastasis in node-negative gastric cancer: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:3927-34. [Crossref] [PubMed]
- Li Y, Du P, Zhou Y, et al. Lymph node micrometastases is a poor prognostic factor for patients in pN0 gastric cancer: a meta-analysis of observational studies. J Surg Res 2014;191:413-22. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- Hsu JT, Yeh TS, Jan YY. Survival impact of the number of lymph node dissection on stage I-III node-negative gastric cancer. Transl Gastroenterol Hepatol 2016;1:9. [Crossref] [PubMed]
Cite this article as: Hsu JT, Le PH, Kuo CJ, Yeh TS, Jan YY. Survival impact of the number of lymph node retrieved on patients with node-negative gastric cancer: more is better? Transl Gastroenterol Hepatol 2017;2:103.


