Pylorus preservation pancreatectomy or not
Introduction
Pancreatic cancer is currently the fourth leading cause of cancer-related deaths in men and women, and has one of the lowest 5-year relative survival rates among all cancer sites—8% at all stages (1). Pancreatic cancer deaths are even projected to increase dramatically to become the second leading cause of death from cancer before 2030 (2). These data illustrate the fatal prognosis of the disease. Complete tumor resection is the only potentially curative option for pancreatic cancer patients and the resection of precursor lesions should be performed at the correct point of time to achieve long-term survival (3).
Pancreatic surgery is a complex, technical procedure regarding diagnostic, surgical and perioperative aspects. Its centralization in specialized institutions has led to acceptable mortality rates below 5% (4,5). Highly standardized surgical techniques and perioperative care are required to achieve low morbidity and mortality rates. However, despite tremendous refinements in the operative technique and better management of complications during the last decades, postoperative morbidity is still as high as up to 50% (6).
Pancreaticoduodenectomy (PD) is the treatment of choice for various benign and malignant tumors of the pancreatic head or the periampullary region. First described by Walther Kausch in Germany in 1909, PD was later refined by Allen O. Whipple in the United States (7,8). The classic Whipple procedure, as it is still performed today, involves en bloc resection of the pancreatic head, the duodenum, the distal common bile duct, the gallbladder, and the distal stomach together with the adjacent lymph-nodes, followed by reconstruction of the gastrointestinal route. In 1944, Watson modified the classic Whipple procedure towards a pylorus-preserving PD in a patient with carcinoma of the papilla of Vater (9). Then a few years later, Traverso and Longmire popularized the preservation of the pylorus in patients with chronic pancreatitis and duodenal cancer (10). Since then, differences in perioperative parameters and postoperative outcomes of classic PD compared to pylorus-preserving PD have been investigated in numerous studies. Avoiding stomach resection without constraining lymph node clearance, pylorus-preserving PD has gained popularity over the classic Whipple procedure in many centers (11,12).
However, there is still a controversial debate on which procedure to perform. Occurring in up to 61% of patients, delayed gastric emptying (DGE) is the most frequent complication after PD (13). One major argument against pylorus preservation has been the hypothesis that the pylorus plays a pivotal role in the pathophysiology of DGE as devascularization and denervation of the pylorus may result in pylorospasm (14-16). In this context, pylorus-resecting PD—synonymously referred to as subtotal stomach-preserving PD—was developed in the late 1990s in Japan. In contrast to the classic Whipple procedure, the entire stomach is preserved in pylorus-preserving and pylorus-resecting PD, and the only difference between both procedures is in whether the pyloric ring is resected or not. The introduction of pylorus-resecting PD to pancreatic surgery has led to further clinical trials investigating the outcomes of PD with or without pylorus resection. During the last 3 years, several primary studies and meta-analyses on this issue have been published illustrating the high relevance of the topic as well as its controversy. The aim of this review was to give a summary of the existing evidence and to answer the question whether to preserve the pylorus or not.
Pylorus-preserving PD versus classic Whipple procedure
Pylorus preservation in PD was initially proposed with the aim to prevent postgastrectomy dumping syndrome and to better preserve the physiological gastrointestinal function with potential benefit on digestion and nutritional status in the long term (17,18). However, opponents of pylorus-preserving PD have questioned its oncological adequacy so that the preservation of the pylorus in cancer patients has been a controversial issue for years. In addition, an increased incidence of DGE was observed in patients undergoing pylorus-preserving PD (19-22). However, depending on trials designs and definitions of outcomes, frequencies of DGE varied considerably between the studies, ranging between 5% and 57% (23).
Postoperative morbidity, mortality and survival
When comparing pylorus-preserving PD and classic Whipple procedure with regard to postoperative morbidity and survival, results from non-randomized studies are highly inconclusive (24-30). With the growing recognition of evidence-based surgery, randomized controlled trials (RCTs) comparing both procedures emerged, followed by systematic reviews and meta-analyses summarizing the existing literature (31-33). Between 1998 and 2015, eight RCTs comparing both procedures in patients with pancreatic and periampullary carcinoma were published (Table 1) (20-22,34-38). A currently updated Cochrane review, gives an excellent summary and critical appraisal of the existing evidence (39). Meta-analyses showed no significant difference between the two procedures regarding mortality, overall survival and relevant parameters of morbidity including pancreatic fistula, postpancreatectomy hemorrhage and biliary leakage (39). The only exception is DGE for which significantly increased rates were shown in patients following pylorus-preserving PD compared to the classic Whipple procedure. In contrast, intraoperative blood loss, operation time, and red blood cell transfusions were significantly reduced in pylorus-preserving PD which may be referred to the lower extent of resection (39).
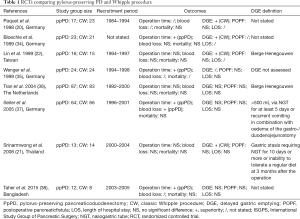
Full table
Limitations of evidence
The validity of meta-analyzed data is limited because of considerable clinical and methodological heterogeneity among the studies included. For instance, inter-study differences in definitions of outcome parameters limit the comparability of trial results. None of the existing RCTs adhered to today’s commonly accepted definition of the International Study Group of Pancreatic Surgery (ISGPS) for DGE (40). This complicates the interpretation of trial results and therefore, conclusions must be drawn with caution. Furthermore, bias results from limitations in trials design and conduct, e.g., lack of blinding and justification of sample size. This is of particular importance in relation to DGE which cannot be regarded as an objective outcome parameter and thus, its evaluation is highly prone to bias within a non-blinded study design (41). The two largest RCTs reported comparable incidences of DGE (36,37), whereas five other RCTs with smaller sample sizes favored the classic Whipple procedure (20-22,34,38). Considering that none of the trials was powered to test a difference in DGE rates, treatment effects may be wrongly estimated.
Overall quality of life was shown to be similar in patients undergoing pylorus-preserving PD and classic Whipple procedure (34,35,37). Moreover, appetite and weight were better preserved in the pylorus-preserving group (34,35). However, data on quality of life and nutritional status are sparse and heterogen owing to different questionnaires used and different time points for follow-up.
Pylorus-preserving versus pylorus-resecting PD
After the introduction of pylorus-resecting PD, Hayashibe and co-workers were the first to report outcomes of pylorus-preserving PD compared to the pylorus-resecting operation in 2007 (42). Since then, several non-randomized and randomized trials on this topic have succeeded. Pylorus resection under preservation of the stomach has been shown to reduce DGE rate significantly in one RCT (43) and six non-randomized comparative trials (42,44-48). In contrast, superiority of pylorus resection was not shown in four other comparative studies including two RCTs (49-52). Hiyoshi et al. evaluated gastric emptying and nutritional status after both procedures during a 12-month period (53). In this study, gastric emptying was evaluated by 13C-acetate breath test before and after surgery. Interestingly, gastric emptying time in the pylorus-preserving group was better preserved than in the pylorus-resecting group when compared to the preoperative function indicating faster gastrointestinal passage after pylorus resection. After body weight decreased significantly in patients in both study groups during the first 6 postoperative months, body weight and body mass index recovered better in the pylorus-preserving group compared to the pylorus-resecting group. The authors concluded that pylorus-preserving PD better preserves physiological gastrointestinal function and long-term nutritional status. However, with only 8 patients in the pylorus-resecting group and 33 patients in the pylorus-preserving group, selection bias must be considered in this study (53).
Resection of the pylorus does not reduce DGE
In the years 2014 and 2015, five meta-analyses summarizing the existing evidence on pylorus-preserving versus pylorus-resecting PD were published, and all of them favored pylorus resection in terms of DGE rate (48,54-57). In contrast, a current blinded RCT including 188 patients failed to show superiority of pylorus resection regarding DGE and other relevant outcome parameters (52). This currently largest RCT was designed and planned with the attempt to overcome the limitations of previous trials with non-blinded trial designs and missing adherence to consensus definitions for outcome parameters. We currently performed a systematic review and meta-analysis to give an update of critically appraised and quantitative data on the effectiveness and safety of pylorus-preserving compared to pylorus-resecting PD (58). Table 2 summarizes the available randomized trials. Meta-analysis of the three existing RCTs showed no significant statistical difference between the two procedures for DGE and other relevant outcome parameters including postoperative pancreatic fistula, postpancreatectomy hemorrhage, intra-abdominal fluid collection/abscess, bile leakage, wound infection, pulmonary complications, mortality, reoperations, perioperative blood loss, duration of operation, and length of hospital stay (58). To account for differences in reconstruction techniques, sensitivity analyses were performed. It was shown that the way of the duodeno-/gastroenteric reconstruction route, i.e., antecolic versus retrocolic reconstruction, and pancreaticojejunostomy versus pancreaticogastrostomy did not substantially alter the results. It was also shown, that the methodological quality of existing non-randomized studies was rather poor. In our own experience, pylorus resection revealed to be superior to pylorus preservation with regard to DGE in a non-randomized series published in 2013 (44), whereas these data could not be confirmed in a previous randomized trial with larger sample size and blinded study design (52).

Full table
Regarding late postoperative outcomes, there is evidence from randomized studies that long-term nutritional and diabetic status, and quality of life are comparable in patients following pylorus-preserving and pylorus-resecting PD (51,59). However, Kawai et al. observed an increased incidence of peptic ulcers following pylorus resection (59). Considering that patients did not undergo routine endoscopy this trend may be underestimated and should be addressed in further studies.
Improvements in methodological quality of surgical trials
In line with the above mentioned randomized trials comparing pylorus-preserving PD and classic Whipple procedure, the existing RCTs comparing pylorus-preserving and pylorus-resecting PD have limitations arising from clinical and methodological heterogeneity, e.g., differences in postoperative management of nasogastric tube removal and start of enteral feeding. Nevertheless, data from latest RCTs illustrate the efforts undertaken to minimize these limitations during the last decade. Regarding DGE and postoperative pancreatic fistula, ISGPS adherence was given in all RCTs comparing pylorus-preserving and pylorus-resecting PD (43,51,52). Moreover, advancements in trial design aspects are obvious. Sample size justification was based on assumptions on the primary endpoint DGE in each study. Blinding of participants, personnel and outcome assessors was reported in one RCT (52)—to give just some of the important improvements.
Future perspectives
While minimal-invasive distal pancreatectomy has gained wide acceptance, open surgery is still the standard approach in PD. Nevertheless, numbers of laparoscopic and robotic PD are increasing in specialized institutions. In a large multi-institutional series including 211 patients undergoing robotic PD and 817 patients undergoing open PD, pylorus-preservation was performed significantly more often in open surgery (60). According to the center’s standard for the open procedure, the pylorus was also preserved in patients undergoing laparoscopic PD (61,62). Wellner et al. showed that laparoscopic pylorus-preserving PD is equivalent to the open procedure regarding postoperative complications, but also showed significantly reduced transfusions and a trend towards shorter operation time, reduced DGE rate, and shorter hospital stay (62). However, long-term outcomes are sparse and further studies are needed to confirm the potential advances of minimal-invasive PD with or without pylorus preservation and to evaluate the oncological adequacy of the procedure in cancer patients.
Summary
Pylorus preservation has gained popularity over the classic Whipple procedure as operation times and intraoperative blood loss were shown to be reduced while relevant short- and long-term outcomes are not affected. Occurring in up to 61% of patients, DGE is the most frequent complication after either procedure. Based on meta-analysis of randomized studies, the classic Whipple procedure is deemed to be superior to pylorus-preserving PD regarding DGE. However, the validity of data is limited as adherence to the ISGPS definition was not given and blinding was lacking in the existing RCTs. This is of particular importance because DGE is prone to bias within non-blinded study designs and missing standards for DGE definition and management. Inter- and intra-study differences in DGE prophylaxis and treatment can further distort trial results. Based on the existing level I evidence studies, there is no convincing benefit of the classic Whipple procedure compared to pylorus-preserving PD—consequently, the pylorus should be preserved whenever possible in patients undergoing pancreatectomy. Considering that pylorus resection does not reduce DGE and long-term data on pylorus-preserving versus pylorus-resecting PD are sparse, removal of the pylorus should no longer be performed as a preventive measure. But, in case of tumor infiltration or concern for sufficient blood supply, pylorus resection in combination with or without distal gastrectomy may be preferred depending on the surgeon’s preference and the individual patient’s situation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Salvia R, Fernández-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg 2004;239:678-85; discussion 685-7. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Harnoss JC, Ulrich AB, Harnoss JM, et al. Use and results of consensus definitions in pancreatic surgery: a systematic review. Surgery 2014;155:47-57. [Crossref] [PubMed]
- Kausch W. Das Carcinom der Papilla duodeni und seine radikale Entfernung. Beitr klin Chir 1912;78:439-86.
- Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg 1935;102:763-79. [Crossref] [PubMed]
- Watson K. Carcinoma of ampulla of vater successful radical resection. Br J Surg 1944;31:368-73. [Crossref]
- Traverso LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet 1978;146:959-62. [PubMed]
- Diener MK, Hüttner FJ, Kieser M, et al. Partial pancreatoduodenectomy versus duodenum-preserving pancreatic head resection in chronic pancreatitis: the multicentre, randomised, controlled, double-blind ChroPac trial. Lancet 2017;390:1027-37. [Crossref] [PubMed]
- Keck T, Wellner UF, Bahra M, et al. Pancreatogastrostomy Versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767): Perioperative and Long-term Results of a Multicenter Randomized Controlled Trial. Ann Surg 2016;263:440-9. [Crossref] [PubMed]
- Eshuis WJ, van Eijck CH, Gerhards MF, et al. Antecolic versus retrocolic route of the gastroenteric anastomosis after pancreatoduodenectomy: a randomized controlled trial. Ann Surg 2014;259:45-51. [Crossref] [PubMed]
- Kim DK, Hindenburg AA, Sharma SK, et al. Is pylorospasm a cause of delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy? Ann Surg Oncol 2005;12:222-7. [Crossref] [PubMed]
- Tanaka A, Ueno T, Oka M, et al. Effect of denervation of the pylorus and transection of the duodenum on acetaminophen absorption in rats; possible mechanism for early delayed gastric emptying after pylorus preserving pancreatoduodenectomy. Tohoku J Exp Med 2000;192:239-47. [Crossref] [PubMed]
- Gauvin JM, Sarmiento JM, Sarr MG. Pylorus-preserving pancreaticoduodenectomy with complete preservation of the pyloroduodenal blood supply and innervation. Arch Surg 2003;138:1261-3. [Crossref] [PubMed]
- Seiler CA, Wagner M, Büchler MW. The role of pylorus-preserving duodenopancreatectomy in pancreatic cancer. Dig Surg 1994;11:378-82.
- Itani KM, Coleman RE, Meyers WC, et al. Pylorus-preserving pancreatoduodenectomy. A clinical and physiologic appraisal. Ann Surg 1986;204:655-64. [Crossref] [PubMed]
- Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, et al. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery 2012;152:S56-63. [Crossref] [PubMed]
- Paquet KJ. Vergleich der partiellen Duodenopankreatektomie (Whipple-Operation) mit der pyloruserhaltenden Zephaloduodenopankreatektomieeine prospektiv kontrollierte, randomisierte Langzeitstudie. Chir gastroenterol 1998;14:54-8.
- Srinarmwong C, Luechakiettisak P, Prasitvilai W. Standard whipple's operation versus pylorus preserving pancreaticoduodenectomy: a randomized controlled trial study. J Med Assoc Thai 2008;91:693-8. [PubMed]
- Lin PW, Lin YJ. Prospective randomized comparison between pylorus-preserving and standard pancreaticoduodenectomy. Br J Surg 1999;86:603-7. [Crossref] [PubMed]
- Traverso LW, Hashimoto Y. Delayed gastric emptying: the state of the highest level of evidence. J Hepatobiliary Pancreat Surg 2008;15:262-9. [Crossref] [PubMed]
- Roder JD, Stein HJ, Hüttl W, et al. Pylorus-preserving versus standard pancreatico-duodenectomy: an analysis of 110 pancreatic and periampullary carcinomas. Br J Surg 1992;79:152-5. [Crossref] [PubMed]
- Jimenez RE, Fernandez-del Castillo C, Rattner DW, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg 2000;231:293-300. [Crossref] [PubMed]
- Warshaw AL, Torchiana DL. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. Surg Gynecol Obstet 1985;160:1-4. [PubMed]
- van Berge Henegouwen MI, van Gulik TM, DeWit LT, et al. Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg 1997;185:373-9. [Crossref] [PubMed]
- Schoenberg MH, Gansauge F, Kunz R. Die Wertigkeit der pyloruserhaltenden partiellen Duodenopankreatektomie beim duktalen Pankreascarcinom. Der Chirurg 1997;68:1262-7. [Crossref]
- Horstmann O, Markus PM, Ghadimi MB, et al. Pylorus preservation has no impact on delayed gastric emptying after pancreatic head resection. Pancreas 2004;28:69-74. [Crossref] [PubMed]
- Belli L, Riolo F, Romani F, et al. Pylorus preserving pancreatoduodenectomy versus Whipple procedure for adenocarcinoma of the head of the pancreas. HPB Surg 1989;1:195-200. [Crossref] [PubMed]
- Diener MK, Knaebel HP, Heukaufer C, et al. A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg 2007;245:187-200. [Crossref] [PubMed]
- Karanicolas PJ, Davies E, Kunz R, et al. The pylorus: take it or leave it? Systematic review and meta-analysis of pylorus-preserving versus standard whipple pancreaticoduodenectomy for pancreatic or periampullary cancer. Ann Surg Oncol 2007;14:1825-34. [Crossref] [PubMed]
- Fitzmaurice C, Seiler CM, Büchler MW, et al. Überleben, Mortalität und Lebensqualität nach pyloruserhaltender oder klassischer Whipple-Operation. Der Chirurg 2010;81:454-71. [Crossref] [PubMed]
- Bloechle C, Broering D, Latuske C, et al. Prospective randomized study to evaluate quality of life after partial pancreatoduodenectomy according to Whipple versus pylorus preserving pancreatoduodenectomy according to Longmire-Traverso for periampullary carcinoma. Dtsch Gesellschaft Chir 1999.suppl 1:661-4. [Crossref]
- Wenger FA, Jacobi CA, Haubold K, et al. Gastrointestinal quality of life after duodenopancreatectomy in pancreatic carcinoma. Preliminary results of a prospective randomized study: pancreatoduodenectomy or pylorus-preserving pancreatoduodenectomy. Chirurg 1999;70:1454-9. [Crossref] [PubMed]
- Tran KT, Smeenk HG, van Eijck CH, et al. Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg 2004;240:738-45. [Crossref] [PubMed]
- Seiler CA, Wagner M, Bachmann T, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg 2005;92:547-56. [Crossref] [PubMed]
- Taher MA, Khan ZR, Chowdhury MM, et al. Pylorus Preserving Pancreaticoduodenectomy vs. Standard Whipple’s Procedure in Case of Carcinoma head of the Pancreas and Periampullary Carcinoma. Mymensingh Med J 2015;24:319-25. [PubMed]
- Hüttner FJ, Fitzmaurice C, Schwarzer G, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 2016;2:CD006053. [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Doerr-Harim C, Bruckner T, Diener MK, et al. Insights into surgical trials: methodological challenges and solutions. Langenbecks Arch Surg 2014;399:273-8. [Crossref] [PubMed]
- Hayashibe A, Kameyama M, Shinbo M, et al. The surgical procedure and clinical results of subtotal stomach preserving pancreaticoduodenectomy (SSPPD) in comparison with pylorus preserving pancreaticoduodenectomy (PPPD). J Surg Oncol 2007;95:106-9. [Crossref] [PubMed]
- Kawai M, Tani M, Hirono S, et al. Pylorus ring resection reduces delayed gastric emptying in patients undergoing pancreatoduodenectomy: a prospective, randomized, controlled trial of pylorus-resecting versus pylorus-preserving pancreatoduodenectomy. Ann Surg 2011;253:495-501. [Crossref] [PubMed]
- Hackert T, Hinz U, Hartwig W, et al. Pylorus resection in partial pancreaticoduodenectomy: impact on delayed gastric emptying. Am J Surg 2013;206:296-9. [Crossref] [PubMed]
- Nanashima A, Abo T, Sumida Y, et al. Comparison of results between pylorus-preserving pancreaticoduodenectomy and subtotal stomach-preserving pancreaticoduodenectomy: report at a single cancer institute. Hepatogastroenterology 2013;60:1182-8. [PubMed]
- Oida T, Mimatsu K, Kano H, et al. Preventing delayed gastric emptying in pancreaticogastrostomy by a modified subtotal-stomach-preserving pancreaticoduodenectomy: Oida modification. Hepatogastroenterology 2011;58:1384-8. [Crossref] [PubMed]
- Fujii T, Kanda M, Kodera Y, et al. Preservation of the pyloric ring has little value in surgery for pancreatic head cancer: a comparative study comparing three surgical procedures. Ann Surg Oncol 2012;19:176-83. [Crossref] [PubMed]
- Zhou Y, Lin L, Wu L, et al. A case-matched comparison and meta-analysis comparing pylorus-resecting pancreaticoduodenectomy with pylorus-preserving pancreaticoduodenectomy for the incidence of postoperative delayed gastric emptying. HPB (Oxford) 2015;17:337-43. [Crossref] [PubMed]
- Kurahara H, Takao S, Shinchi H, et al. Subtotal stomach-preserving pancreaticoduodenectomy (SSPPD) prevents postoperative delayed gastric emptying. J Surg Oncol 2010;102:615-9. [Crossref] [PubMed]
- Akizuki E, Kimura Y, Nobuoka T, et al. Prospective nonrandomized comparison between pylorus-preserving and subtotal stomach-preserving pancreaticoduodenectomy from the perspectives of DGE occurrence and postoperative digestive functions. J Gastrointest Surg 2008;12:1185-92. [Crossref] [PubMed]
- Matsumoto I, Shinzeki M, Asari S, et al. A prospective randomized comparison between pylorus- and subtotal stomach-preserving pancreatoduodenectomy on postoperative delayed gastric emptying occurrence and long-term nutritional status. J Surg Oncol 2014;109:690-6. [Crossref] [PubMed]
- Hackert T, Probst P, Knebel P, et al. Pylorus Resection Does Not Reduce Delayed Gastric Emptying After Partial Pancreatoduodenectomy: A Blinded Randomized Controlled Trial (PROPP Study, DRKS00004191). Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Hiyoshi M, Chijiiwa K, Ohuchida J, et al. Comparative study of gastric emptying and nutritional status after pylorus-preserving vs. subtotal stomach-preserving pancreaticoduodenectomy. Hepatogastroenterology 2012;59:1018-22. [PubMed]
- Hanna MM, Gadde R, Tamariz L, et al. Delayed Gastric Emptying After Pancreaticoduodenectomy: Is Subtotal Stomach Preserving Better or Pylorus Preserving? J Gastrointest Surg 2015;19:1542-52. [Crossref] [PubMed]
- Huang W, Xiong JJ, Wan MH, et al. Meta-analysis of subtotal stomach-preserving pancreaticoduodenectomy vs pylorus preserving pancreaticoduodenectomy. World J Gastroenterol 2015;21:6361-73. [Crossref] [PubMed]
- Wu W, Hong X, Fu L, et al. The effect of pylorus removal on delayed gastric emptying after pancreaticoduodenectomy: a meta-analysis of 2,599 patients. PLoS One 2014;9:e108380. [Crossref] [PubMed]
- Chen QJ, He ZQ, Yang Y, et al. Is there comparable morbidity in pylorus-preserving and pylorus-resecting pancreaticoduodenectomy? A meta-analysis. J Huazhong Univ Sci Technolog Med Sci 2015;35:793-800. [Crossref] [PubMed]
- Klaiber U, Probst P, Strobel O, et al. Meta-analysis of delayed gastric emptying after pylorus-preserving versus pylorus-resecting pancreatoduodenectomy. Br J Surg 2017. [Epub ahead of print].
- Kawai M, Tani M, Hirono S, et al. Pylorus-resecting pancreaticoduodenectomy offers long-term outcomes similar to those of pylorus-preserving pancreaticoduodenectomy: results of a prospective study. World J Surg 2014;38:1476-83. [Crossref] [PubMed]
- Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg 2016;264:640-9. [Crossref] [PubMed]
- Kim SC, Song KB, Jung YS, et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc 2013;27:95-103. [Crossref] [PubMed]
- Wellner UF, Küsters S, Sick O, et al. Hybrid laparoscopic versus open pylorus-preserving pancreatoduodenectomy: retrospective matched case comparison in 80 patients. Langenbecks Arch Surg 2014;399:849-56. [Crossref] [PubMed]
Cite this article as: Klaiber U, Probst P, Büchler MW, Hackert T. Pylorus preservation pancreatectomy or not. Transl Gastroenterol Hepatol 2017;2:100.


