Function-preserving surgery for gastric cancer: current status and future perspectives
Introduction
In the Japanese gastric cancer treatment guidelines, standard gastrectomy is defined that the resection of at least two-thirds of the stomach with a D2 lymph node dissection is needed (1). Conversely, function-preserving gastrectomy (FPG), which maintains gastric function to the detriment of the advantages that standard gastric cancer surgery provides, is performed to address the post-operative quality of life (QOL) of the patients. Although evidently not defined in the guidelines, FPG is characterized as a procedure that preserves the esophagogastric junction, pylorus, and capacity of the remnant stomach to maintain a functional reservoir (2,3). Pylorus-preserving gastrectomy (PPG) and proximal gastrectomy (PG), two representative FPG procedures, are generally performed with a curative intent in early gastric cancer (EGC) according to limited indications. A more limited gastric resection approach, i.e., local resection of the stomach and segmental gastrectomy, are not generally performed, as it is not a radical procedure for gastric cancer; endoscopic resection has been established as an alternative procedure as a localized treatment in these cases.
FPG, which was adapted for EGC patients, was shown to achieve favorable prognosis with reduced surgical invasiveness, and its indications overlap with those of laparoscopic gastrectomy, a form of minimally invasive surgery (2-4). Indeed, FPG was often performed laparoscopically in recent years. Optional procedures including the preservation of vagal nerve are often performed together with FPG, although their benefits remain unclear.
In this review, we will summarize the current landscape of FPG with curative intent in gastric cancer, including PPG, PG, distal gastrectomy (DG) with a very small remnant stomach, and local resection of the stomach.
PPG
PPG, originally utilized for the surgical treatment of gastric peptic ulcers with satisfactory results, has since been introduced as a surgical treatment for EGC to preserve function and maintain a better QOL (5-8). PPG is generally considered to offer several advantages over conventional DG with Billroth I reconstruction including lower incidence rates of dumping syndrome, duodenal juice reflux, and altered bowel habits; maintenance of body weight; and reduced flatus frequency (9-11).
Indications of PPG
Indications of PPG are cT1N0 EGC located in the middle one-third of the stomach. Distance of the lesion from pylorus should be at least 5 cm. At our institute, strict criteria including tumor size, histology of the tumor, age, and body mass index (BMI) are not employed for PPG. As to the other eligibility of criteria, patients with hiatal-hernia and difficult dietary restrictions would not meet the criteria of PPG, because the possibility of reflux after surgery would be high. In our database, the rate of metastasis to suprapyloric region nodes was only 0.2% in 3,646 patients with T1 (mucosal or submucosal) cancer located in gastric midbody (12). Therefore, PPG is indicated for the patients with cT1 gastric cancer who meet the other eligibility criteria.
Laparoscopic procedures in PPG
Laparoscopic gastrectomy for EGC, which was first reported by Kitano et al., is performed widely in Asian countries (13). In Japan, laparoscopic gastrectomy is performed in more than 10,000 cases per year, as revealed by a national survey. Laparoscopy-assisted PPG (LAPPG) is increasingly preferred as it is associated with a lower incidence of postoperative pain, earlier return of bowel functions, shorter periods of hospitalization, and improved cosmetic results (Figure 1) (6,7). In a recent retrospective our study of 307 patients with EGC, we reported LAPPG as a safe approach with excellent postoperative outcomes including reductions in major complications based on the Clavien-Dindo classification, which focuses mainly on the therapeutic consequences of complications and has been shown as a reliable tool for quality assessment in surgery (11). In that study, major complications greater than Clavien-Dindo classification IIIa, were observed in only 4 patients (1.3%). The most frequent complication was gastric stasis, which occurred in 19 patients (6.2%). We further identified in the reports that high BMI and lower surgical experience with LAPPG were significant risk factors for postoperative complications.
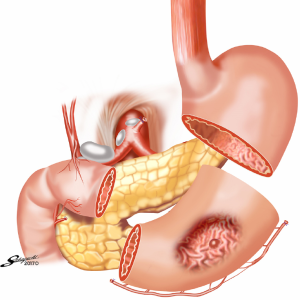
During LAPPG, anastomosis can be easily performed directly from the small upper abdominal middle incision, as the site for anastomosis site is immediately under the incision for hand-sewn procedures. In reports of complete intracorporeal anastomosis during LAPPG, delta-shaped anastomosis was performed with demonstrated feasibility and safety in initial experiences (Figure 2) (14,15). Conversely, reports of intracorporeal end-to-end anastomosis after LAPPG were also published, where anastomosis was performed using a combination of a stapler and a hand-sewn procedure (16).
Oncological safety of PPG
Several retrospective reports with relatively a great many patients who underwent PPG consistently showed the oncological safety of PPG (17-21). PPG procedures that were evaluated those that preserved the nerve system and blood flow supply to the pyloric antrum for maintenance of the pyloric function, which resulted in incomplete lymph node dissection of the suprapyloric and infrapyloric lymph nodes. Although previous reports suggested that the probability of metastasis to the suprapyloric and infrapyloric lymph nodes from EGC located in gastric midbody as negligible, such limited lymph node dissection approaches during PPG might increase the risk of recurrence (22,23).
The 5-year survival rates for EGC after gastrectomy with radical lymph node dissection were reported ranges from 93% to 98% (24,25). Recently, several studies evaluating survival rates with PPG for EGC reported that 96% to 98% 5-year survival rates (17,19), which were clearly in line with previous reports on mortality rates for EGC.
While an overall 3-year survival rate of 97.8% and a disease-specific 3-year survival rate of 99.3% in 188 patients treated by LAPPG were reported by Jiang et al., there is ongoing debate on whether PPG indications should always be applied for EGC and whether the prognosis of conventional DG is superior to PPG in these patients (18). Randomized controlled trials should resolve these clinical questions, and the Korean Laparoscopic Gastrointestinal Surgery Study (KLASS)-04, a multicenter randomized controlled trial which has been recruiting patients since July 2015, will determine the superiority of LAPPG to laparoscopic DG in postoperative QOL and survival rates of patients with EGC in the gastric midbody. Additionally, we recently reported the comparison of oncological outcomes with PPG and conventional DG for EGC in a multicenter propensity score-matched cohort analysis (20). Our findings indicated that the oncological safety of PPG for clinical T1N0 gastric cancer in the middle portion of the stomach was comparable to that of DG in cases with correctly estimated clinical tumor stage by the statistical method.
Gastric stasis after PPG
PPG prevents postprandial symptoms such as dumping syndrome and alkaline reflux (9). However, delayed gastric retention due to aberrant pyloric function can occur during the early postoperative period in 23% to 40% of the patients who underwent PPG (26,27). Patients who underwent PPG sometimes report the feeling of gastric fullness, and some patients exhibit long-term retention of food in the residual stomach; however, some investigators claim that the vagal nerve and the infrapyloric artery should be preserved to prevent postoperative stasis, which is experienced by 6% to 8% of the patients (7,11). Specifically related to the rate of gastric stasis after LAPPG, the infrapyloric vein should prevent pyloric cuff edema, thus minimizing the incidence of gastric stasis; a study indeed investigated early clinical outcomes of LAPPG with preservation of the infrapyloric vein, not only infrapyloric artery (28). The incidence of both gastric stasis and transient delayed gastric emptying was significantly lower in patients who underwent LAPPG that preserved the infrapyloric artery and vein than in those who underwent LAPPG without the preservation of the infrapyloric vein. Conversely, length of the remaining pyloric cuff appears to have a certain degree of impact on postoperative outcomes after PPG, as severe postoperative edema of the pyloric cuff might impact gastric wall motility after PPG.
The length of the remnant pyloric cuff, which was initially preserved at 1.5 cm, has been increased to 3 cm or more in PPG procedures in recent years (5). However, Morita et al. reported that there were no pronounced differences in several functional parameters and symptoms after PPG between patients with a remnant pyloric cuff of less than 3 cm and those with a remnant pyloric cuff of more than 3 cm (29).
A multicenter survey-based study in Japan, which utilized the newly developed postgastrectomy syndrome assessment scale (PGSAS)-45, found that factors including a sufficient proximal gastric remnant minimized postgastrectomy symptoms following PPG (30).
PG
With the recently increasing incidence of proximal gastric cancer in Asian countries (31-34), PG is widely accepted as a PPG in EGC (35-37). Some authors also argue that PG is not oncologically and functionally preferred to total gastrectomy for EGC that is located at the upper stomach. Total gastrectomy with extended lymphadenectomy (D2) is firmly established as the standard approach for gastric cancer involving the upper third of the stomach in Japan. Analysis of outcomes in patients with EGC treated by the standard Japanese D2 total gastrectomy in the 1980s showed that nodal metastasis in distal perigastric lymph nodes was rarely recognized (36); therefore, dissection of these nodes was considered not to be necessary. The Japanese Gastric Cancer Guideline for the treatment of gastric cancer in 2014 also recommended modified procedures including PG for cT1N0 gastric cancer as a surgical treatment option (1). Since the first report by Uyama et al. in 1995, laparoscopic PG has been increasingly performed, with several technical reports and small sample-sized case studies already published (38-42). Conversely, PG is expected to preserve the reservoir function of the remnant distal stomach including the pyloric ring function that prevents duodenogastric reflux and to be associated with a lower rate of dumping syndrome (43-46). Patients who undergo PG may also suffer from heartburn or gastric fullness resulting in esophageal reflux, which could lead to a poor postoperative QOL (31,47).
Reconstruction after PG
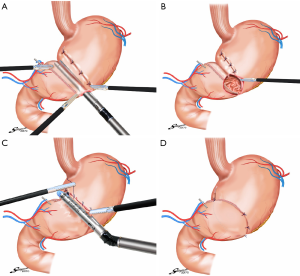
Several reconstruction methods can be adopted after PG, including esophagogastrostomy (38,39,45,46), jejunal interposition (JI) (43,44), and double-tract reconstruction (40,48). Esophagogastrostomy is the simplest reconstruction method; however, it is associated with a high risk of reflux esophagitis and gastroesophageal anastomotic stenosis (47,49). Tokunaga et al. conducted a questionnaire survey to evaluate these subjective symptoms after PG and determined that while JI might prevent endoscopic gastroesophageal reflux, it was also associated with higher incidence rates of subjective symptoms indicating delayed emptying (50). Thus, the authors concluded that EG was a superior reconstruction method based on subjective symptoms and length of surgery. However, several issues remain to be resolved including endoscopic esophagitis during the postoperative period. In that regard, a promising reconstruction method after PG was laparoscopically performed recently in Japan; esophagogastrostomy was performed using a hand-sewn procedure, and the esophagogastric junction was reconstructed with the double-flap technique to prevent reflux (Figures 3-6) (45,46). The original procedure that formed the basis for this method was designed for conventional open surgery. At our institute, we had favorable outcomes including morbidity and nutritional status after laparoscopic PG with the double-flap method compared with LATG (45).
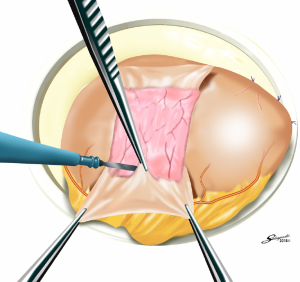
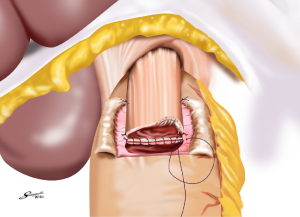
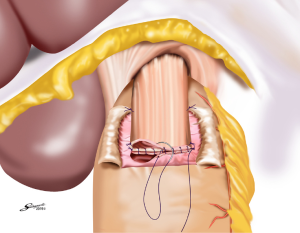
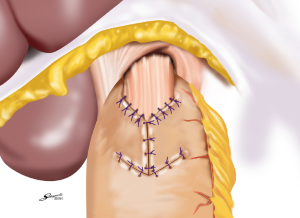
JI has several issues as well, which include technical complexity, association with functional disorders indicating delayed emptying and difficulty in endoscopic surveillance, particularly in patients with longer interposed segments (50).ouble-tract reconstruction is another complicated function-preserving procedure that remains unproven in potential functional advantages, especially the reservoir function (40). The KLASS-05 trial in Korea compares laparoscopic proximal gastrectomy (LAPG) with double-tract reconstruction and LTG. Primary endpoint is the change in hemoglobin level at 2-year postgastrectomy, and secondary endpoints are incidence rates of postoperative reflux esophagitis and anastomotic stricture, incidence of morbidity and mortality, QOL at two-year postgastrectomy, and three-year disease-free survival.
Finally, optimal reconstruction approaches following PG are a topic of ongoing debate.
Gastric cancer in the remnant stomach after PG
An important consideration in PG is the increased risk for remnant gastric cancer. The rate of remnant gastric cancer is higher after PG (3.6–9.1%) than that reported after DG (0.4–2.5%) (51-54). Aggressive endoscopic screening in asymptomatic patients leads to early detection and curative resection of gastric cancer in the remnant stomach (55-57). Intubation of endoscopy after esophagogastrostomy, which is not difficult, can be a challenging procedure after esophagojejunostomy, especially in patients with a longer interposed segment (50,58). Evaluation of the remnant stomach in patients with an interposed jejunum greater than 10 cm in length remains challenging; therefore, surgeons performing PG with JI reconstruction should pay attention to the length of the interposed jejunal when considering endoscopic follow-up.
Subtotal gastrectomy with very small remnant stomach
With the increasing number of patients with EGC in the upper third of the stomach, laparoscopic total gastrectomy and PG are performed as minimally invasive surgical approaches. However, these procedures carry a high level of technical difficulty, especially if laparoscopic reconstruction is coupled with esophagojejunostomy or esophagogastrostomy. Higher postoperative anastomotic complication rates were also reported for LATG compared with LADG (59). Meanwhile, loss of the esophagogastric junction after LATG or LAPG is associated with esophageal reflux, which can have a significant effect on QOL. Jiang et al. evaluated the utility of laparoscopic subtotal gastrectomy with a very small remnant stomach in patients with EGC in the upper third stomach, in whom the distance between the proximal margin of the tumor and the esophagogastric junction was at least 3 cm (60). We also evaluated the feasibility and the nutritional impact of LAsTG in comparison with LATG (61). We found that LAsTG was a better choice than LATG for EGC in the upper stomach due to improvements in the incidence of anastomotic complications and postoperative nutritional status. However, preserving a very small remnant stomach for EGC in the upper stomach raises several concerns including oncological safety and reconstruction techniques available for gastrojejunostomy with a limited remnant stomach.
Indication and oncological safety of LAsTG
The indications for LAsTG are: (I) EGC that was preoperatively diagnosed as cT1N0, located in or involving the upper third of the stomach; (II) less than 5 cm distance between the tumor and the esophagogastric junction, and the less than 3 cm distance between the remnant gastric stump and the esophagogastric junction. During tumor evaluation in patients who will undergo LAsTG, preoperative endoscopy is performed, and the proximal tumor margin should be marked by endoscopic clips and be confirmed as tumor-negative by step biopsy. In cases with a positive proximal margin biopsy, LATG would be selected instead of LAsTG.
Lymphatic flow from a tumor located in the upper stomach, especially in the posterior wall and the greater curvature, sometimes drains to lymph nodes located distal to the splenic artery via the posterior gastric artery and the splenic hilum. Therefore, tumors in the lesser curvature of the upper stomach fulfilling the abovementioned indications are predicted to be associated with favorable outcomes with LAsTG, as nearly all lymph nodes in the lesser curvature of the stomach can be dissected even during LAsTG, without any arterial sacrifice.
Reconstruction techniques for gastrojejunostomy after LAsTG
Reconstruction methods to achieve gastrojejunostomy are another obstacle during LAsTG. Roux-en-Y gastrojejunostomy is the only available reconstruction procedure, as reflux after gastrectomy can be effectively avoided even if the size of the remnant stomach is very small. Side-to-side gastrojejunostomy during Roux-en-Y reconstruction was usually performed with 60-mm linear staplers in reported studies on open or laparoscopic gastrectomy. However, Roux-en-Y reconstruction using a 60-mm stapler requires a relatively large remnant stomach and sufficient blood supply from the short gastric artery due to the anastomotic site. Conversely, in patients with a very small remnant stomach, some of the short gastric arteries may be interrupted by stapling; therefore, a 45-mm stapler or a circular stapler should be used in LAsTG. We often utilize a modified technique with the OrVil system and a 25-mm circular stapler (Premium Plus CEEA stapler; Covidien Japan, Tokyo, Japan), which is a simple and time-saving technique during anastomosis in cases with a narrow anastomosis site in the remnant stomach. Embedding of the anvil rod in the greater curvature of the gastric stump is an important aspect of this technique, as it allows for the formation of a safe anastomosis with minimal tension.
Local resection of the stomach
Endoscopic submucosal dissection (ESD) is accepted as a less invasive local resection approach in EGC cases that carry a negligible risk of lymph node metastasis (62,63). Based on the estimated risk of lymph node metastasis in EGC determined by a large number of surgical cases (64,65), the criteria for ESD in EGC has been expanded to include larger lesions of intramucosal cancer (>21 mm in diameter) or ulcerated lesions. However, ESD is still a complicated procedure, and the expanded indications for ESD were found to be associated with higher incidence rates of bleeding and perforation, particularly in large EGC lesions, as well as longer operation times (66-68).
Before the development of ESD, some patients with EGC underwent local resection of the stomach using a lesion-lifting technique (69-73). However, indications for this approach rapidly decreased due to problems with determination of the exact tumor dissection line while maintaining safe cutting margins; ESD subsequently became the main treatment of EGC (70). Theoretically, dissection of any lesion is possible with ESD, regardless of the size of the tumor or the presence of ulcerative changes. However, ESD remains a complicated technique that requires a high level of skill from endoscopists, especially for larger lesions. Difficulty in reaching the gastric wall and collection of fluids including blood and/or gastric juices hinder ESD performance and results in low rates of complete removal of EGC lesions as intact specimens (67,68).
With the aim to appropriately resect the gastric wall, we developed the laparoscopic and endoscopic cooperative surgery (LECS) technique for the dissection of submucosal tumors of the stomach (74). In LECS, first, tumor location is confirmed endoscopically, which is followed by submucosal dissection using intraluminal endoscopy to determine the appropriate resection line. Next, the seromuscular layer is dissected laparoscopically, and the incision line is closed using a laparoscopic stapling device. Nunobe et al. used LECS in a case with laterally spreading intramucosal gastric cancer that fulfilled the extended criteria of ESD according to Japanese gastric cancer treatment guidelines (Figures 7-9) (1,75).
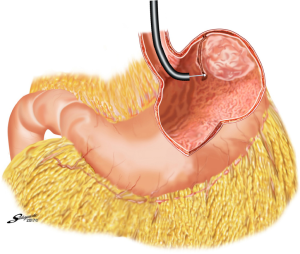
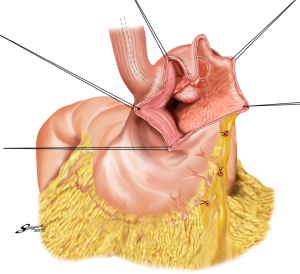
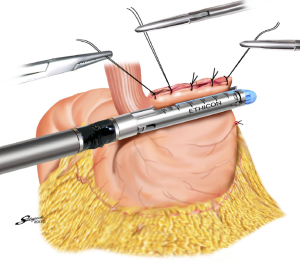
With LECS for epithelial neoplasms, it is critical to ensure that no tumor cells are seeded in the peritoneal cavity. To prevent any contact with the visceral tissue, tumor is turned towards the intragastric cavity by traction on the stiches at the edge of the resected specimen, and resection line of the stomach is pulled up like a bowel by several stiches. Only gastric perforation during ESD for gastric cancer has been reported not to lead to peritoneal dissemination even with long-term observation (76).
To reduce the risk of cancer cell seeding through the open gastric wall, several full-thickness gastric wall resection approaches utilizing non-exposure techniques such as the CLEAN-NET and the NEWS have been developed (77,78). CLEAN-NET achieves full-thickness resection of the stomach wall using only laparoscopy and is followed by endoscopy to confirm the dissection line. Conversely, NEWS utilizes endoscopy to assist the laparoscopic approach. However, as the mucosal layer shifts significantly from the seromuscular layer during surgery, the muscular and the seromuscular layers may be incorrectly dissected using the CLEAN-NET and NEWS techniques. Indications of LECS techniques, including inverted LECS, CLEAN-NET and NEWS, for EGC might be extended in the future, with establishment of sentinel lymph node assessment for surgical treatment of gastric cancer. This approach should result in decreased failure rates of gastrectomy in the treatment of gastric cancer.
In summary, FPG has the potential to increase QOL in EGC. However, several issues such as precise estimation of preserved function, confirmation of oncological safety, and standardized techniques for FPG including laparoscopic procedures need to be strictly addressed. Therefore, there is a need for several well-designed, prospective cohort and randomized studies.
Acknowledgements
The authors are deeply grateful to Shigeyuki Sakaguchi for drawing illustrations.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- Hiki N, Nunobe S, Kubota T, et al. Function-Preserving Gastrectomy for Early Gastric Cancer. Ann Surg Oncol 2013;20:2683-92. [Crossref] [PubMed]
- Nomura E, Okajima K. Function-preserving gastrectomy for gastric cancer in Japan. World J Gastroenterol 2016;22:5888-95. [Crossref] [PubMed]
- Kitano S, Shiraishi N, Uyama I, et al. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 2007;245:68-72. [Crossref] [PubMed]
- Maki T, Shiratori T, Hatafuku T, et al. Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery 1967;61:838-45. [PubMed]
- Hiki N, Shimoyama S, Yamaguchi H, et al. Laparoscopy-assisted pylorus-preserving gastrectomy with quality controlled lymph node dissection in gastric cancer operation. J Am Coll Surg 2006;203:162-9. [Crossref] [PubMed]
- Nunobe S, Hiki N, Fukunaga T, et al. Laparoscopy-assisted pylorus-preserving gastrectomy: preservation of vagus nerve and infrapyloric blood flow induces less stasis. World J Surg 2007;31:2335-40. [Crossref] [PubMed]
- Nishikawa K, Kawahira H, Yumiba T, et al. Functional characteristics of the pylorus in patients undergoing pylorus-preserving gastrectomy for early gastric cancer. Surgery 2002;131:613-24. [Crossref] [PubMed]
- Nunobe S, Sasako M, Saka M, et al. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer 2007;10:167-72. [Crossref] [PubMed]
- Fujita J, Takahashi M, Urushihara T, et al. Assessment of postoperative quality of life following pylorus-preserving gastrectomy and Billroth-I distal gastrectomy in gastric cancer patients: results of the nationwide postgastrectomy syndrome assessment study. Gastric Cancer 2016;19:302-11. [Crossref] [PubMed]
- Jiang X. Postoperative outcomes and complications after laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Ann Surg 2011;253:928-33. [Crossref] [PubMed]
- Nakajima T, Yamaguchi T. editors. The gastric cancer registry database 1946-2004 (Cancer Institute Hospital gastric cancer database 1946-2004). Tokyo: Kanehara Pub Ltd, 2006.
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Kumagai K, Hiki N, Nunobe S, et al. Totally laparoscopic pylorus-preserving gastrectomy for early gastric cancer in the middle stomach: technical report and surgical outcomes. Gastric Cancer 2015;18:183-7. [Crossref] [PubMed]
- Lee SW, Bouras G, Nomura E, et al. Intracorporeal stapled anastomosis following laparoscopic segmental gastrectomy for gastric cancer: technical report and surgical outcomes. Surg Endosc 2010;24:1774-80. [Crossref] [PubMed]
- Koeda K, Chiba T, Noda H, et al. Intracorporeal reconstruction after laparoscopic pylorus-preserving gastrectomy for middle-third early gastric cancer: a hybrid technique using linear stapler and manual suturing. Langenbecks Arch Surg 2016;401:397-402. [Crossref] [PubMed]
- Hiki N, Sano T, Fukunaga T, et al. Survival benefit of pylorus-preserving gastrectomy in early gastric cancer. J Am Coll Surg 2009;209:297-301. [Crossref] [PubMed]
- Jiang X, Hiki N, Nunobe S, et al. Lomg-term outcome and survival with laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc 2011;25:1182-6. [Crossref] [PubMed]
- Morita S, Katai H, Saka M, et al. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg 2008;95:1131-5. [Crossref] [PubMed]
- Aizawa M, Honda M, Hiki N, et al. Oncological outcomes of function-preserving gastrectomy for early gastric cancer: a multicenter propensity score matched cohort analysis comparing pylorus-preserving gastrectomy versus conventional distal gastrectomy. Gastric Cancer 2017;20:709-17. [Crossref] [PubMed]
- Tsujiura M, Hiki N, Ohashi M, et al. Excellent Long-Term Prognosis and Favorable Postoperative Nutritional Status After Laparoscopic Pylorus-Preserving Gastrectomy. Ann Surg Oncol 2017;24:2233-40. [Crossref] [PubMed]
- Kong SH, Kim JW, Lee HJ, et al. The safety of the dissection of lymph node station 5 and 6 in pylorus-preserving gastrectomy. Ann Surg Oncol 2009;16:3252-8. [Crossref] [PubMed]
- Kim BH, Hong SW, Kim JW, et al. Oncologic safety of pylorus-preserving gastrectomy in the aspect of micrometastasis in lymph nodes at stations 5 and 6. Ann Surg Oncol 2014;21:533-8. [Crossref] [PubMed]
- Sano T, Sasako M, Kinoshita T, et al. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer 1993;72:3174-8. [Crossref] [PubMed]
- Sue-Ling HM, Johnston D, Martin IG, et al. Gastric cancer: a curable disease in Britain. BMJ 1993;307:591-6. [Crossref] [PubMed]
- Tomita R, Fujisaki S, Tanjoh K. Pathophysiological studies on the relationship between postgastrectomy syndrome and gastric emptying function at 5 years after pylorus-preserving distal gastrectomy for early gastric cancer. World J Surg 2003;27:725-33. [Crossref] [PubMed]
- Kodama M, Koyama K, Chida T, et al. Early postoperative evaluation of pylorus-preserving gastrectomy for gastric cancer. World J Surg 1995;19:456-60. [Crossref] [PubMed]
- Kiyokawa T, Hiki N, Nunobe S, et al. Preserving infrapyloric vein reduces postoperative gastric stasis after laparoscopic pylorus-preserving gastrectomy. Langenbecks Arch Surg 2017;402:49-56. [Crossref] [PubMed]
- Morita S, Sasako M, Saka M, et al. Correlation between the length of the pyloric cuff and postoperative evaluation after pylorus-preserving gastrectomy. Gastric Cancer 2010;13:109-16. [Crossref] [PubMed]
- Namikawa T, Hiki N, Kinami S, et al. Factors the minimize postgastrectomy symptoms following pylorus-preserving gastrectomy: assessment using a newly developed scale (PGSAS-45). Gastric Cancer 2015;18:397-406. [Crossref] [PubMed]
- Ahn HS, Lee HJ, Yoo MW, et al. Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. Br J Surg 2011;98:255-60. [Crossref] [PubMed]
- Blaser MJ, Saito D. Trends in reported adenocarcinomas of the oesophagus and gastric cardia in Japan. Eur J Gastroenterol Hepatol 2002;14:107-13. [Crossref] [PubMed]
- Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer 2011;14:301-16. [Crossref] [PubMed]
- Zhou Y, Zhang Z, Zhang Z, et al. A rising trend of gastric cardia cancer in Gansu Province of China. Cancer Lett 2008;269:18-25. [Crossref] [PubMed]
- Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery 1998;123:127-30. [Crossref] [PubMed]
- Katai H, Sano T, Fukagawa T, et al. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg 2003;90:850-3. [Crossref] [PubMed]
- Takiguchi N, Takahashi M, Ikeda M, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multiinstitutional study. Gastric Cancer 2015;18:407-16. [Crossref] [PubMed]
- Uyama I, Ogiwara H, Takahara T, et al. Laparoscopic and minilaparotomy proximal gastrectomy and esophagogastrostomy: technique and case report. Surg Laparosc Endosc 1995;5:487-91. [PubMed]
- Ichikawa D, Komatsu S, Okamoto K, et al. Esophagogastrostomy using a circular stapler in laparoscopy-assisted proximal gastrectomy with an incision in the left abdomen. Langenbecks Arch Surg 2012;397:57-62. [Crossref] [PubMed]
- Ahn SH, Jung DH, Son SY, et al. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 2014;17:562-70. [Crossref] [PubMed]
- Kitano S, Adachi Y, Shiraishi N, et al. Laparoscopic-assisted proximal gastrectomy for early gastric carcinomas. Surg Today 1999;29:389-91. [Crossref] [PubMed]
- Sakuramoto S, Yamashita K, Kikuchi S, et al. Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg 2009;209:344-51. [Crossref] [PubMed]
- Katai H, Morita S, Saka M, et al. Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg 2010;97:558-62. [Crossref] [PubMed]
- Kinoshita T, Gotohda N, Kato Y, et al. Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: a retrospective comparison with open surgery. Surg Endosc 2013;27:146-53. [Crossref] [PubMed]
- Hayami M, Hiki N, Nunobe S, et al. Clinical Outcomes and Evaluation of Laparoscopic Proximal Gastrectomy with Double-Flap Technique for Early Gastric Cancer in the Upper Third of the Stomach. Ann Surg Oncol 2017;24:1635-42. [Crossref] [PubMed]
- Kuroda S, Nishizaki M, Kikuchi S, et al. Double-Flap Technique as an Antireflux Procedure in Esophagogastrostomy after Proximal Gastrectomy. J Am Coll Surg 2016;223:e7-13. [Crossref] [PubMed]
- An JY, Youn HG, Choi MG, et al. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg 2008;196:587-91. [Crossref] [PubMed]
- Nomura E, Lee SW, Kawai M, et al. Function outcomes by reconstruction technique following laparoscopic proximal gastrectomy for gastric cancer: double tract versus jejunal interposition. World J Surg Oncol 2014;12:20. [Crossref] [PubMed]
- Ronellenfitsch U, Najmeh S, Andalib A, et al. Functional outcomes and quality of life after proximal gastrectomy with esophagogastrostomy using a narrow gastric conduit. Ann Surg Oncol 2015;22:772-9. [Crossref] [PubMed]
- Tokunaga M, Ohyama S, Hiki N, et al. Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: comparison between esophagogastric anastomosis and jejunal interposition. World J Surg 2008;32:1473-7. [Crossref] [PubMed]
- Nozaki I, Hato S, Kobatake T, et al. Long-term outcome after proximal gastrectomy with jejunal interposition for gastric cancer compared with total gastrectomy. World J Surg 2013;37:558-64. [Crossref] [PubMed]
- Kikuchi S, Nemoto Y, Katada N, et al. Results of follow-up endoscopy in patients who underwent proximal gastrectomy with jejunal interposition for early gastric cancer. Hepatogastroenterology 2007;54:304-7. [PubMed]
- Ohyama S, Tokunaga M, Hiki N, et al. A clinicopathological study of gastric stump carcinoma following proximal gastrectomy. Gastric Cancer 2009;12:88-94. [Crossref] [PubMed]
- Nozaki I, Kurita A, Nasu J, et al. Higher incidence of gastric remnant cancer after proximal than distal gastrectomy. Hepatogastroenterology 2007;54:1604-8. [PubMed]
- Greene FL. Management of gastric remnant carcinoma based on the results of a 15-year endoscopic screening program. Ann Surg 1996;223:701-6. [Crossref] [PubMed]
- Newman E, Brennan MF, Hochwald SN, et al. Gastric remnant carcinoma: just another proximal gastric cancer or a unique entity? Am J Surg 1997;173:292-7. [Crossref] [PubMed]
- Kaneko K, Kondo H, Saito D, et al. Early gastric stump cancer following distal gastrectomy. Gut 1998;43:342-4. [Crossref] [PubMed]
- Iwata T, Kurita N, Ikemoto T, et al. Evaluation of reconstruction after proximal gastrectomy: prospective comparative study of jejunal interposition and jejunal pouch interposition. Hepatogastroenterology 2006;53:301-3. [PubMed]
- Lee SE, Ryu KW, Nam BH, et al. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol 2009;100:392-5. [Crossref] [PubMed]
- Jiang X, Hiki N, Nunobe S, et al. Laparoscopy-assisted subtotal gastrectomy with very small remnant stomach: a novel surgical procedure for selected early gastric cancer in the upper stomach. Gastric Cancer 2011;14:194-9. [Crossref] [PubMed]
- Kosuga T, Hiki N, Nunobe S, et al. Feasibility and nutritional impact of laparoscopy-assisted subtotal gastrectomy for early gastric cancer in the upper stomach. Ann Surg Oncol 2014;21:2028-35. [Crossref] [PubMed]
- Rembacken BJ, Gotoda T, Fujii T, et al. Endoscopic mucosal resection. Endoscopy 2001;33:709-18. [Crossref] [PubMed]
- Soetikno RM, Gotoda T, Nakanishi Y, et al. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567-79. [Crossref] [PubMed]
- Eguchi T, Gotoda T, Oda I, et al. Is endoscopic one-piece mucosal resection essential for early gastric cancer? Dig Endosc 2003;15:113-6. [Crossref]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-25. [Crossref] [PubMed]
- Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005;17:54-8. [Crossref]
- Gotoda T, Oda I, Tamakawa K, et al. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos). Gastrointest Endosc 2009;69:10-5. [Crossref] [PubMed]
- Rösch T, Sarbia M, Schumacher B, et al. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy 2004;36:788-801. [Crossref] [PubMed]
- Ludwig K, Klautke G, Bernhard J, et al. Minimally invasive and local treatment for mucosal early gastric cancer. Surg Endosc 2005;19:1362-6. [Crossref] [PubMed]
- Ohgami M, Otani Y, Kumai K, et al. Curative laparoscopic surgery for early gastric cancer: five years experience. World J Surg 1999;23:187-92. [Crossref] [PubMed]
- Hiki Y, Sakuramoto S, Katada N, et al. Combined laparoscopic-endoscopic procedure in stomach carcinoma. Chirurg 2000;71:1193-201. [Crossref] [PubMed]
- Kobayashi T, Kazui T, Kimura T. Surgical local resection for early gastric cancer. Surg Laparosc Endosc Percutan Tech 2003;13:299-303. [Crossref] [PubMed]
- Seto Y, Yamaguchi H, Shimoyama S, et al. Results of local resection with regional lymphadenectomy for early gastric cancer. Am J Surg 2001;182:498-501. [Crossref] [PubMed]
- Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008;22:1729-35. [Crossref] [PubMed]
- Nunobe S, Hiki N, Gotoda T, et al. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer 2012;15:338-42. [Crossref] [PubMed]
- Ikehara H, Gotoda T, Ono H, et al. Gastric perforation during endoscopic resection for gastric carcinoma and the risk of peritoneal dissemination. Br J Surg 2007;94:992-5. [Crossref] [PubMed]
- Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am 2012;21:129-40. [Crossref] [PubMed]
- Goto O, Mitsui T, Fujishiro M, et al. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer 2011;14:183-7. [Crossref] [PubMed]
Cite this article as: Nunobe S, Hiki N. Function-preserving surgery for gastric cancer: current status and future perspectives. Transl Gastroenterol Hepatol 2017;2:77.


