Selection of patients with hepatocellular cancer: a difficult balancing between equity, utility, and benefit
Ethical principles used for selecting patients and allocating grafts in liver transplantation (LT)
LT represents the gold-standard strategy in patients having a hepatocellular cancer (HCC), offering a potential cure for both the tumour and the underlying liver cirrhosis. However, LT effectiveness is limited by the scarcity of donors (1). As a consequence, different selection policies were proposed with the intent to select the best candidates for LT (1).
Two major ethical principles are used in the process of allocating grafts to recipients with end-stage hepatic illness: equity and utility (2). Equity includes: (I) horizontal equity = equal treatments for equal needs; and (II) vertical equity = prioritization of patients presenting the worst clinical conditions (3,4). Horizontal equity suggests that groups of recipients presenting different diseases (for example, HCC vs. non-tumoral pathologies) should receive identical priority. Vertical equity corresponds to the urgency principle of “the sickest first”.
On the opposite, utility is connected with the concept of utilitarianism. As a consequence, if on one side transplanting “the sickest first” consents to prioritize cases with the worst survivals if left untreated, an allocation process based on the concept of utility consents to save the greatest number of life-years (2).
Ethical principles influence the selection process for LT in different ways and moments.
At the moment of LT indication, LT selection corresponds to inclusion and exclusion criteria for enrolling patients in a specific waiting list (WL).
During the WL period, the selection process corresponds with the decision about the priority of patients (i.e., the order they have in the WL) and when dropping out patients for disease progression. At the moment of organ allocation, LT selection corresponds to decide who among candidates in the WL should receive that specific graft (1).
Main endpoints used to describe ethical principles for LT patient selection
Looking at a selection process based on the concept of urgency, recipients expected to experience the poorest outcomes during the waiting time period receive the highest priority for LT (5-7). The main endpoints used to describe vertical equity—urgency in LT, therefore, are the risk of death during the WL period or the risk of drop-out (DO) from the WL for disease progression before LT. For cases having non-malignant (NM) hepatic diseases, well-known scores aimed at estimating liver function, like the model for end-stage liver disease (MELD) and the Child-Turcotte-Pugh scores, have been translated in the WL reality to measure the priority for LT (5-7). Recently, use of MELD score has importantly shortened the mean WL duration in the United States (8), endorsing worldwide as the most common method to describe the priority of patients with NM liver cirrhosis (9).
As for utility-based system is concerned, the main utility endpoint considered in recent years is the expected post-transplant outcome measured as post-LT overall survival or post-LT disease free survival (7).
Selection policies for tumoral recipients waiting for a transplant are mainly based on this simplified utility endpoint (10-12). In fact, the scarce survivals reported in the first series of LT for HCC recipients favoured the development of more severe selection criteria [i.e., Milan Criteria (MC)] principally focused on outcomes after transplantation (12). Therefore, tumours exceeding MC were excluded from the possibility of being treated with a LT. Furthermore, in the currently used allocation systems, all patients having a tumour within MC present the same priority despite they should present an overall different risk of dying during the WL period (i.e., which depends on liver function, tumour stage, and availability of alternative therapies).
If patients waiting for transplantation having vs. not having tumour are considered as two distinct groups, selection and allocation principles currently used worldwide appear to be apparently opposite. This discrepancy finally ends in an ethical paradox: livers are transplanted to the “sickest first” patient among the NM recipients, whilst the “earliest first” patient is transplanted among the HCC ones, no matter on their potential survivals using therapies other than LT.
Starting from these considerations (i.e., increasing the percentage of HCC cases in the WL, and reducing the excess of priority for recipients with tumour having a low urgency for transplant), several scenarios have been proposed with the intent to reduce the great discrepancy in LT access existent among patients having HCC vs. NM liver disease. The development of risk models within the HCC population was attempted, aiming at identify the risk of DO at three months as a common urgency end-point (1). Nonetheless, such an approach (i.e., compare the DO risk in different types of LT recipient) should cause an excessive prioritization of patients with tumour presenting higher biological aggressiveness (i.e., greater tumours, higher AFP levels), thus dramatically increasing post-transplant death and tumour recurrence rates (1). As a consequence, it is fundamental to identify methods able to create a balancing between the principles of urgency and utility, having as final objective the intent to reach equity among HCC vs. NM recipients.
The most correct endpoint to describe the utility principle is the “transplant benefit” (TB). TB represents the survival gain obtained by transplantation by comparison with the best alternative therapies (i.e., difference between life years obtained with and without LT) (1).
However, the survival benefit-based allocation process recently reported in some studies (6,7) does not always coincide with the ethical concept of utility, depending upon the time horizon (13).
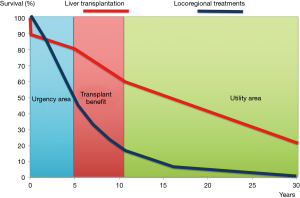
Time horizon represents the estimated length of time in which a specific event (death, recurrence) is observed. Typically, articles do not report the duration of time horizon: however, the decision of a specific time horizon presents profound ethical implications. When TB is estimated using a long-term horizon (>10 years), it strongly reflects a “pure utility” allocation model (post-LT outcomes) (Figure 1). In this case, TB suffers from several biases like “ageism” and poor long-term predictive ability (2).
On the opposite, when TB presents a short time horizon (one to three years), it is strongly determined by variables able to predict non-LT survivals (MELD, tumour staging, alternative strategies), obtaining a “pure urgency” measurement, with an increased risk of reporting “futile” LT deriving from transplanting very sick recipients with predicted poor post-transplant survivals (13).
TB: from utility endpoint to ideal allocation principle
A post-transplant time horizon placed between five and ten years is contemporaneously influenced by pre- and post-LT covariates: such a situation explains why Schaubel defines TB as a good balancing “between urgency and utility” (7). On this perspective, the TB used with a mid-term time horizon, has the intrinsic potential to reach the dignity of an independent LT selection principle.
The great advantage of this principle is its ability to cover the entire process of LT, contemporaneously being able to consider pre and post-transplant outcomes.
Furthermore, the TB principle is superior respect to urgency and utility also from a population perspective, consenting to prioritize recipients using the life-years gained thanks to LT (7). Such superiority derives from the fact that an urgency-based system tends to allocate grafts to recipients with a greater risk of death during the waiting time period; however, this approach is connected with a contemporaneous detriment of utility, because patients having a higher risk of dying during the waiting time period also present the highest risk of post-transplant mortality.
On the opposite, a utility-based allocation system consents to transplant patients having the lowest risk of dying after LT; however, recipients presenting the best survivals after transplantation typically present better survivals also during the waiting time period. Thus, the TB principle looks to be best tool for maximizing the total number of life-years obtained by the patients thanks to transplantation.
TB principle was initially investigated in transplant recipients (6,7), however only focusing on NM recipients or considering HCC as a complication and not as a separate and prognostically heterogeneous clinical pathology. On the opposite, TB looks to be extremely useful for recipients with tumour, being expressed also in terms of gain in life expectancy (LE), a value obtained after the subtraction of the survival curve obtained after alternative curative strategies from the survival curve obtained after LT (12).
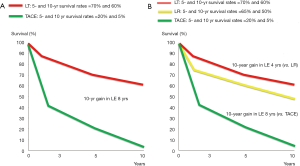
Such an aspect gives great importance not only to the crude survivals after transplantation, but also to other fundamental HCC prognosticators, like the presence of available alternative strategies (14). Two different clinical scenarios are shown in Figure 2. In the first case, a 40-year old HCC recipient with a tumour burden within the MC presents a 5-year estimated survival rate after liver transplant of 70%. The absence of alternative therapies able to obtain acceptable results finally determines a very high TB (Figure 2A). The second case reports a 65-year old recipient with similar estimated post-LT survivals (5-year =70%), but with an effective alternative therapy (hepatectomy), thus making the TB inferior respect to the first case, despite LT remains the best option in both the scenarios (Figure 2B).
Recently published papers focused on the different survivals of patients having advanced tumours after transplantation vs. alternative therapeutic strategies (11,15-18) consented to estimate the TB also in HCC exceeding MC. From a utility point of view, enlisting patients with tumour extended criteria would mean allocating a greater number of livers to these recipients respect to non tumoral ones (19). On the other hand, looking at this situation from a TB perspective, would mean allocating the same number of grafts to different groups of patients obtaining an overall increase in benefit. In other terms, the TB principle would consent to increase the total life-years of tumoral and non tumoral patients taken together.
TB in the different phases of LT selection for HCC patients
The TB principle can be first used to decide which patients should be included in the WL for LT. These concepts have been recently incorporated in several papers. (11,15,16,18,20-22) Three different aspects are focused in these studies: (I) LT consents to obtain the best survival benefit in tumoral cases contemporaneously having an advanced cirrhosis (BCLC stage D); (II) when effective alternative strategies lack, patients having a BCLC stage B–C obtain an important TB, even in case of patients exceeding MC; (III) when effective alternative strategies are present, patients with early tumour and compensated cirrhosis present the lowest TB.
When an HCC has been already included in the WL, the TB can be used also to decide his/her priority for LT (i.e., his/her position in the WL) with respect with other HCC patients and those with NM cirrhosis. We recently proposed an HCC MELD formula able to equate the priority of patients with and without HCC in a common WL by using the TB principle (10).
This formula has been recently included in the Italian score for allocation (22).
At the moment of graft allocation, the concept of TB has also the potential to take into account donor characteristics in order to optimize the match between donor and recipients in similar positions of the WL (7).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Toso C, Mazzaferro V, Bruix J, et al. Toward a better liver graft allocation that accounts for candidates with and without hepatocellular carcinoma. Am J Transplant 2014;14:2221-7. [Crossref] [PubMed]
- Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet 2009;373:423-31. [Crossref] [PubMed]
- Brock DW, Wikler D. Chapter 14: Ethical Issues in Resource Allocation, Research, and New Product Development. In: DT, Breman JG, Measham AR, et al. editors. Disease Control Priorities in Developing Countries. 2nd edition. New York: Oxford University Press, 2006.
- James C, Carrin G, Savedoff W, et al. Clarifying efficiency-equity tradeoffs through explicit criteria, with a focus on developing countries. Health Care Anal 2005;13:33-51. [Crossref] [PubMed]
- Toso C, Majno P, Berney T, et al. Validation of a dropout assessment model of candidates with/without hepatocellular carcinoma on a common liver transplant waiting list. Transpl Int 2014;27:686-95. [Crossref] [PubMed]
- Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant 2005;5:307-13. [Crossref] [PubMed]
- Schaubel DE, Guidinger MK, Biggins SW, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant 2009;9:970-81. [Crossref] [PubMed]
- Ioannou GN, Perkins JD, Carithers RL Jr. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology 2008;134:1342-51. [Crossref] [PubMed]
- Freeman RB Jr. The model for end-stage liver disease comes of age. Clin Liver Dis 2007;11:249-63. [Crossref] [PubMed]
- Vitale A, Volk ML, De Feo TM, et al. A method for establishing allocation equity among patients with and without hepatocellular carcinoma on a common liver transplant waiting list. J Hepatol 2014;60:290-7. [Crossref] [PubMed]
- Vitale A, Morales RR, Zanus G, et al. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol 2011;12:654-62. [Crossref] [PubMed]
- Cillo U, Vitale A, Volk ML, et al. The survival benefit of liver transplantation in hepatocellular carcinoma patients. Dig Liver Dis 2010;42:642-9. [Crossref] [PubMed]
- Vitale A, Volk M, Cillo U. Urgency, utility, and time horizon of transplant benefit. Liver Transpl 2015;21:565-6. [Crossref] [PubMed]
- Vitale A, Volk M, Cillo U. Transplant benefit for patients with hepatocellular carcinoma. World J Gastroenterol 2013;19:9183-8. [Crossref] [PubMed]
- Berry K, Ioannou GN. Are patients with Child's A cirrhosis and hepatocellular carcinoma appropriate candidates for liver transplantation? Am J Transplant 2012;12:706-17. [Crossref] [PubMed]
- Vitale A, Huo TL, Cucchetti A, et al. Survival Benefit of Liver Transplantation Versus Resection for Hepatocellular Carcinoma: Impact of MELD Score. Ann Surg Oncol 2015;22:1901-7. [Crossref] [PubMed]
- Spolverato G, Vitale A, Ejaz A, et al. The relative net health benefit of liver resection, ablation, and transplantation for early hepatocellular carcinoma. World J Surg 2015;39:1474-84. [Crossref] [PubMed]
- Vitale A, Farinati F, Burra P, et al. Utility-based criteria for selecting patients with hepatocellular carcinoma for liver transplantation: A multicenter cohort study using the alpha-fetoprotein model as a survival predictor. Liver Transpl 2015;21:1250-8. [Crossref] [PubMed]
- Toso C, Kneteman NM, James Shapiro AM, et al. The estimated number of patients with hepatocellular carcinoma selected for liver transplantation using expanded selection criteria. Transpl Int 2009;22:869-75. [Crossref] [PubMed]
- Cillo U, Vitale A, Polacco M, et al. Liver transplantation for hepatocellular carcinoma through the lens of transplant benefit. Hepatology 2017;65:1741-8. [Crossref] [PubMed]
- Lai Q, Vitale A, Iesari S, et al. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Cillo U, Burra P, Mazzaferro V, et al. A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a "Blended Principle Model". Am J Transplant 2015;15:2552-61. [Crossref] [PubMed]
Cite this article as: Vitale A, Lai Q. Selection of patients with hepatocellular cancer: a difficult balancing between equity, utility, and benefit. Transl Gastroenterol Hepatol 2017;2:75.