Using a weaning immunosuppression protocol in liver transplantation recipients with hepatocellular carcinoma: a compromise between the risk of recurrence and the risk of rejection?
Introduction
In the last years hepatocellular carcinoma (HCC) recurrence after liver transplantation (LT) has attracted great attention in the transplant community. Despite a careful selection of candidates for LT and optimization of bridging loco-regional therapies within the waiting list, HCC recurrence rate is still up to 15–20%, despite a 5-year survival rate of 70% after LT (1,2).
In order to improve the outcome of HCC recipients, in the last decades different criteria have been proposed in order to stage the pre-LT HCC status and its related prognosis after transplantation. These include the Milan Criteria and its implementation, the University of California San Francisco criteria, the Barcelona-Clinic Liver Cancer criteria, the Asan Medical Center criteria, the University Clinic of Navarra’s criteria and the Kyoto criteria (3).
HCC recurrence has been associated with several “oncological” risk factors, such as compliance to Milan and other criteria, tumour size, Edmondson-Steiner histology grades, the vascular and capsular invasion and alpha-fetoprotein blood levels (4,5), as well as several “non-oncological” risk factors, including immunosuppressive (IS) drugs, type of donor (living-donor vs. deceased-donor), graft-related variables (e.g., ischemia-reperfusion injury), recipient characteristics (body mass index, age) and waiting-list time to LT (6). The principal mechanisms of HCC recurrence are considered related to the sub-clinical extrahepatic spread of tumour at time of LT and/or nesting of tumoral cells during the manipulation of the liver during surgery.
The current strategies to minimize HCC recurrence after LT are mainly based on (I) a careful selection of candidates with HCC for LT; (II) the optimization of patient management within the waiting list (through the prioritization and bridging strategies); (III) the administration of a tailored IS regimen in the post-transplant period.
Nowadays, the identification of the optimal IS regimen in HCC recipients is one of the main goals after LT. IS therapy is essential in preventing graft rejection, however presenting a well-established association with oncogenesis (2). The immune system has a key role as a defending mechanism against cancer development, preventing vascular invasion and/or metastasis and its depression has been largely associated with de novo and recurrent malignancies (7). Therefore, the relation between tumour recurrence and the type of IS therapy is crucial in HCC recipients, representing one of the few risk factors modifiable after LT.
A tailored IS regimen is essential to gain the optimal balance between the risk of rejection and the risk of HCC recurrence. So far, a complete withdrawal of IS drugs after LT has been reported to be safely achievable in 25% of patients (defined as “operational tolerant”), without the risk of graft loss (8). IS weaning has the benefit of reducing IS-related side effects such as infections, diabetes, renal failure, cardiovascular diseases and de novo tumours (9-11). Therefore, in HCC-LT recipients, IS withdrawal could theoretically contribute to reduce the risk of tumour recurrence, which represents the major drawback in these recipients.
Herein, we review the current literature on IS weaning in recipients who underwent LT for HCC as primary indication and we report our experience on IS withdrawal in HCC recipients.
Immunosuppression drugs and HCC recurrence
Calcineurin inhibitors (CNIs) are the mostly used IS drugs after solid organ transplantation. Its introduction has improved patient and graft outcomes; however, CNIs have shown to be a risk factor for HCC recurrence. CNIs promote the proliferation of malignant cells in a dose-dependent fashion through the increase of angiogenesis and cancer cell invasiveness, activation of pro-oncogenes, and enhance tumour growth factor-β (TGF-β) expression, inducing resistance to apoptosis and increasing the likelihood of metastasis (12). Several retrospective studies reported that HCC recurrence is related to CNIs’ dose-exposure: high doses of CNIs (mean trough concentrations of tacrolimus >10 ng/mL or cyclosporine >300 ng/mL) during the first postoperative period exerted a negative impact on HCC recipients with higher tumour recurrence rates (12).
In recent years, the introduction of IS regimens based on mammalian target of rapamycin inhibitors (mTORi), sirolimus and everolimus, provided a new potential tool to prevent HCC recurrence after LT. The mTOR pathway, in fact, plays a central function in the cellular metabolism, growth and proliferation and its up-regulation is present in up to 50% of HCC tumours (13).
Sirolimus was the first developed mTORi and its potential anti-tumour effect has been documented by several studies, improving the overall patient survival rate in HCC recipients (14,15). A recent meta-analysis showed that sirolimus-based IS regimens decrease tumour recurrence and improve LT outcomes, with no significant difference in major post-transplant complications (16). In 2014, a systematic review of 3,666 HCC LT patients from 42 studies reported that recipients treated with CNIs developed HCC recurrence significantly more frequently, compared to those receiving mTORi (13.8% vs. 8%, P<0.001). Moreover, patients under everolimus had significantly lower recurrence rates of HCC, compared with those on sirolimus or CNIs (4.1% vs. 10.5% vs. 13.8%, respectively, P<0.05) (17).
Despite the above consideration, more recently, the results from the SiLVER study definitely showed that LT recipients treated with mTORi were not superior in terms of recurrence free survival (18).
Antimetabolite drugs have a controversial role in preventing HCC recurrence. Long-term use of azathioprine increases the risk of lymphoma and non-melanoma skin cancer, but there is no evident effect on HCC (19). So far, mycophenolate did not show any effect on HCC recurrence, despite it seems to be protective against other malignancies after transplantation (20,21).
Recently, other immune-modulator drugs such as sorafenib and belacept, has been associated with the conventional IS regimens after LT with promising results, but further data are needed (22,23).
Moreover, new specific immunotherapies such as dendritic cell vaccination and immune checkpoint inhibition are under investigation for the treatment of HCC after loco-regional treatment and could potentially be used also in the post-transplant setting (24).
Operational tolerance after liver transplantation
The status of “tolerance” after a solid organ transplantation is defined as a “functioning graft without histological sign of rejection in the absence of IS and in an immunocompetent host that can accept a second graft from the same donor but reject a graft from a different third-party donor” (25). In other words, tolerance is the non-reactivity of immune system, that has not been suppressed by IS, to one or more antigens. In LT, the term of clinical operational tolerance (COT) is defined as a “transplant retaining function without features of acute or chronic rejection in the absence of any IS” (26).
The mechanisms of spontaneous allograft acceptance in LT recipients are not completely clear yet. The liver is less likely to be rejected than other solid transplanted organs and a number of mechanisms, in order to explain this peculiarity, have been proposed: (I) the liver seems to produce high levels of soluble major histocompatibility complex (MHC) that could impact in recipients’ immune response (27); (II) the donor liver appears to present hematopoietic stem cells influencing the recipients’ chimerism (28); (III) during LT a number of donor liver leukocytes migrate into the recipient blood stream and this could contribute to the graft’s tolerance, as their depletion by irradiation in the donor led to organ rejection (29); (IV) the size of the liver, which is approximately 10 times greater than other transplantable organs (heart, kidney or pancreas), can act as a cytokine sink and/or dilute the clones of alloreactive T cells and thus potentially exhaust the recipients’ immune response; (V) the liver is a unique organ for its microenvironment and microcirculation since it is constantly exposed to different antigens deriving from intestine bacteria and/or food and this could be responsible for a major acceptance of allograft rather than other organs.
The first series of COT in LT was described by the Pittsburgh group in the early 1990s, reporting 11 recipients who completely withdrew IS due to non-compliance or post-transplant lymphoproliferative disorders (30). Later, the same group designed a prospective trial in which 28 out of 95 (29%) recipients were successfully weaned off IS within a long-term follow-up (31).
The King’s College group reported also a similar experience: out of 18 recipients in which IS withdrawal was attempted, 5 (28%) patients were completely weaned off from IS drugs, but only 2 were able to remain in an IS-free status in the long-term (32).
In 2006, investigators from the Transplant Unit of Tor Vergata University described the results of 34 LT recipients with recurrent hepatitis C virus (HCV)-related disease in whom IS weaning was attempted: among these patients, complete IS drugs weaning was successfully achieved in 8 (23.4%) patients, while the reminders who couldn’t complete the IS withdrawal process were returned to the initial regimen without any case of graft loss (33). After a 10-year follow-up, 6 (17.6%) tolerant patients persisted in an IS-free status, without any signs of rejection, with normal liver function tests (LFTs) and lower incidence of IS drug-related side-effects (such as recurrent infection and new onset of diabetes mellitus) (10).
More recently, in 2013, the European Consortium of transplant tolerance published the results of a prospective multicenter trial of IS withdrawal in 102 adult LT recipients of cadaveric liver grafts. In this trial, 41 (40%) reached the primary endpoint, defined as a stable biochemical and histological graft status for at least 1 year after complete IS drugs discontinuation (34).
In the last 10 years, 7 major COT trials have been reported in LT, which are summarized in Table 1. A total of 285 tolerant recipients have been investigated, including 20 (7%) paediatric patients (<18 years) transplanted with living donor grafts from consanguineous donors (33-39). In most trials, common inclusion criteria were stable graft function for at least 1 year and absence of autoimmune disease; protocol graft biopsies were performed before starting the weaning process to exclude histological signs of graft rejection. The mean follow-up from LT to complete IS withdrawal was 74 (range: 48–112) months. COT was achieved from 22% up to 62.5% of recipients who attempted withdrawal of IS drugs. The incidence of acute rejection was higher in HCV-positive LT recipients, while it was less frequent in the paediatric population transplanted with living-donor grafts (76% vs. 10%, respectively).
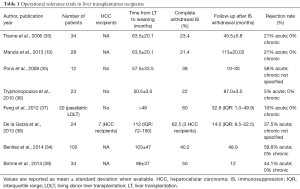
Full table
These studies demonstrate that tolerant recipients present stable graft function after IS weaning in the long-term follow-up and that complete withdrawal of IS drugs could be safely achieved in more than one quarter of carefully selected LT recipients without compromising patients and grafts survival (9,40).
Predicting factors of tolerance
Mostly, the aim of the COT trials was to define the “predicting factors of tolerance”, which could identify LT recipients who could successfully withdraw IS therapy after transplantation. So far, the main “clinical predictors of tolerance” which have been recognized include: (I) a long follow-up period after LT (minimal time of 3 years from transplantation to IS weaning); (II) absence of autoimmune disease; (III) older LT recipients (>60 years old).
Moreover, the IS regimen after transplantation seems to play also a role in the development of COT. The use of CNIs at low-dose during the first post-transplant weeks is considered as an independent factor for successful IS-weaning due to its interference in the cytokine microenvironment, in the pathway of T-cell activation and T-receptor down-regulation. However, the immunological mechanism of this finding is still not completely clear (41).
In order to identify a priori which patient could be potentially tolerant after LT, researchers focused on the assessment of the immunological signatures peculiar of tolerant and non-tolerant recipients through the identification of “biomarkers”. The latter are commonly defined as “objective and measurable indicators of physiological processes, pathological conditions, or pharmacological responses” (42). The “biomarkers of tolerance” were mainly explored in peripheral blood using multi-parameter flow cytometry, microarrays or real-time PCR gene expression profiling, immunophenotypic and genotypic pathway of peripheral blood mononuclear cells (PBMCs).
In 2003, the Pittsburgh group quantified the human antigen presenting cells (APC)-dendritic cell subsets in 6 LT tolerant patients and in 23 non-tolerant recipients. Data showed that the ratio between monocytoid dendritic cells, inducing Th1 responses, and plasmacytoid dendritic cell, inducing Th2 responses, was significantly lower in the tolerant group (43). Later, other studies, analysing the immunophenotyping of the PBMCs, found that there was an increasing number of CD4+, CD25+ and increased ratio γδ1/γδ2 T-cells in tolerant patients (44,45).
Moreover, recently a regulator of CD4+ and CD25+ T-reg cells, named FOXP3 gene was found to be strongly associated to the tolerance status when detected in peripheral blood samples (35).
All these findings suggest that the detection of “biomarkers of tolerance” in peripheral blood could help identifying patients who can discontinue IS therapy. Notably, all the above studies are based on a limited number of patients and therefore further clinical trials with larger population samples are needed to confirm these results.
HCC and tolerance: state of the art
So far, there are only few data in the literature on the achievement of a stable IS-free status in patients who underwent LT for HCC.
Induction of tolerance to the liver graft after bone marrow transplantation was investigated in the last years. In 2004, the Ghent-Brussels group described two patients who received a right lobe graft from a living donor for malignancy (1 HCC and 1 cholangiocarcinoma), after CD34+ stem cells harvested from the same donor (46). In these series, the main aim was to attempt IS withdrawal after LT. The post-operative IS protocol consisted of steroids, rapamycin, and anti-thymocyte globulin; IS drugs were discontinued after 90 and 28 days from LT, without subsequent rejection episodes. In the two cases liver function remained stable. The HCC patient died due to tumour recurrence after 370 days, while the other was still alive after 270 days, but with a suspected cholangiocarcinoma relapse. Despite this experience being limited to only two cases, this clinical report showed that acceptance of human leukocyte antigen (HLA) mismatched living-donor liver graft after non-myeloablative conditioning and donor stem cell infusion permits to achieve a tolerant status and hence could potentially reduce the risk of tumour recurrence by improving the rate of immune reconstitution. Later, the same group proposed a less drastic conditioning regimen (anti-thymocyte globulin and sirolimus combination) achieving a state of donor-specific tolerance in other four patients. Since these additional case series has only a short follow-up reported [5.3±1.8 (range, 3.5–8) months], the data do not allow to draw conclusions, yet they suggest that further investigations in the field are needed.
The only cases of COT without induction in HCC-LT patients were reported by De la Garza et al. in 2013 (38): in their trial including a total of 24 patients, 7 (29%) recipients underwent LT for HCC. After a mean time of 112 (range, 72–160) months from IS weaning, 3 (43%) HCC recipients presented a tolerant status, while 4 (57%) patients had to reassume IS drugs. However, no data on HCC recurrence status after IS weaning was reported.
HCC and tolerance: the Tor Vergata experience
Since 2004, investigators form the Liver Unit of the University of Tor Vergata have conducted researches on the minimization and discontinuation of IS therapy after LT (7,10,11,32,33). In our clinical practice, the post-transplant IS protocol is based on a tailored immunosuppressant regimen with the aim to (I) minimize IS therapy within 1 year after LT (we usually adopt low-dose CNI and steroid-free regimen from the first weeks after transplantation); (II) attempt IS drugs’ discontinuation after the 3th years from LT, in those patients with stable LFTs, who didn’t experience previous rejection episodes and/or autoimmune disease. During the IS tapering LFTs and clinical examination are routinely performed every 4 weeks during drug’s tapering and subsequently every 3 months for the first year of the weaning process. In case of increases in LFTs, above 2-fold the normal levels no further decrease in drug dosages are scheduled, and additional LFTs are being performed within 14 days. Worsening or persistence of biochemical alterations is an indication for IS resumption at the same dose before the weaning process was started. In case of persistent LFTs abnormality, a liver biopsy is usually performed.
In our institution, a total of 113 LT attempted IS discontinuation between 2001 and 2016: among these, 37 (32.7%) patients successfully discontinued IS therapy after LT.
HCC was the primary indication for LT in 17 patients [14 M/3 F, median age at LT was 55.5 (range, 43–65) years old] who attempted IS withdrawal and their clinical details are reported in Table 2. Development of HCC was associated with a pre-LT diagnosis of HCV infection in 10 (58.8%) cases, hepatitis B virus (HBV) infection in 2 (11.7%), alcohol-related cirrhosis in 3 (17.6%), co-infection of HBV-HCV or HBV-HDV virus in 2 (11.7%). The median time from LT to IS withdrawal was 7.6 (range, 2–15) years. At the time of IS initiation of weaning, the mean recipient age was 63.2 (range, 47–72) years old, 16 (94.1%) patients were receiving CNI-based regimens, while 1 (5.9%) were treated with m-TORi based therapy. The mean time to wean off IS therapy was 6 (range, 2–9) months. After a median follow-up of 25.8 (range, 14–73) months, 7 (41.1%) LT recipients completely discontinued IS drugs and reached a tolerant status, with stable LFTs. At the last follow-up, the mean serum level of aspartate aminotransferase (AST) was 24 (range, 14–38) U/L, alanine aminotransferase (ALT) was 23 (range, 17–53) U/L, Total bilirubin was 0.7 (range, 0.3–1.4) mg/dL, without clinical and biochemical signs of rejection.
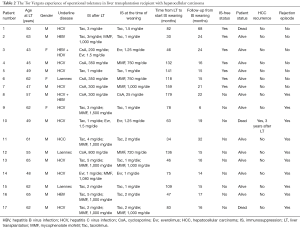
Full table
Ten patients (58.9%) were transiently tolerant, defined as those LT recipients who presented signs of rejection (LFTs above 2-fold normal levels) after IS discontinuation; these patients required IS resumption within 5.6 (range, 3–10) months from drug cessation, followed by complete recovery of graft function and normalization of serum biochemistry. No case of non-tolerant patients has been reported [defined as LT recipients who presented signs of rejection (LFTs above 2-fold normal levels) during IS minimization]. No graft and patient loss was observed in the entire follow-up. Among HCC tolerant group, no evidence of HCC recurrence was observed, while 1 (5.88%) transiently tolerant patient presented HCC recurrence after 3 years from transplantation and died within 4 years.
Discussion
The role of IS therapy after LT in patients with HCC is of paramount importance, in order to avoid acute and chronic rejection while protecting the recipients from tumour recurrence. The relationship between IS and HCC recurrence after LT seems to be evident. The available IS regimens inhibit the immune system at many different levels simultaneously, weakening both the adaptive and innate immune response. This results in a higher susceptibility to tumour relapse and de novo malignancy after LT.
With the currently available IS regimens, the best approach to avoid HCC recurrence would be to minimize the number and dosage of drugs and to administrate tailored IS regimen for HCC recipients as soon as possible after LT. Several studies showed that CNIs’ decrease should be universally applied at short-term after LT (12,17).
The recent SiLVER study reported that mTORi-based IS regimens have a favorable impact on tumour recurrence in the first 5 years post LT but seems to not improve the recurrence free state beyond 5 years (18). Definitely results are awaited from an ongoing prospective randomised open-labelled clinical trial to better understand mTORi effects on tumour recurrence (NCT00355862, available at: http://www.clinicaltrials.gov). Yet, despite these initial evidences, the best IS regimen for patients with HCC is still under debate (47).
Since a certain degree of immune response is desirable to prevent tumour relapse, theoretically, the goal for recipients who receive LT for HCC should be a “state of tolerance”. An IS-free status could potentially on one hand reduce the risk of tumour recurrence, while, on the other hand, have a favorable effect on patient outcomes (40). The long-term benefits of IS weaning process have been extensively reported: withdrawing IS therapy is associated with decreasing drug’s related side-effects such as cardiovascular diseases, infections, de novo tumours, new onset diabetes and dyslipidaemia, as well as maintaining a better post-transplant recipients’ compliance without compromising graft and patient survival (40).
A “state of tolerance” can be successfully achieved in about 25% of carefully selected LT. As mentioned above, results from multicentre trials conducted in Europe and the United States in adult and paediatric patients have shown that COT can be achieved more often than originally estimated. Clinical predictor factors, such as stable graft function, length of follow-up after LT, IS type and blood levels, close matching of HLA type between donor and recipient, absence of autoimmune liver diseases, no history of rejection, were used as inclusion criteria for patient selection (33-39).
Long-term results of IS weaning have shown that the longer the follow-up after LT the higher the likelihood of weaning off IS, consequently most authors prefer to attempt weaning 12–36 months after LT (33-34).
Since some important clinical predictors of COT have been identified, in recent years clinicians and scientists have focused their attention on determining the immunological signature of the tolerant recipients with the aim of identifying those patients who could successfully be weaned off IS. The development of non-invasive “biomarker of tolerance” testing in the setting of LT, mainly based on examination of blood samples, currently assumes an important role in HCC LT recipients due to the increasing likelihood of identifying COT recipients who might be able to undergo a reduction of IS or be withdrawn from it. The principal role of such biomarkers is to evaluate the immune status of recipients, either phenotypic or genotypic, and to identify those associated with COT (42).
In HCC recipients the use of “biomarkers of tolerance” might assume a critical role in order to identify which patients might potentially early weaned off IS. As it is well known, most of HCC recurrence occurs within 2 years after LT, while the IS minimization and/or withdrawal is challenging in the early post-transplant period for the risk of rejection. Consequently, the use of “biomarkers of tolerance” to select patients for drugs withdrawal might be crucial. It is conceivable that recipients who underwent transplantation for HCC outside the Milan criteria might particularly benefit from IS weaning off, since the risk of a single episode of acute rejection early after LT is easily managed, while tumour relapse has limited therapeutic options.
The Tor Vergata experience is the largest reported so far on COT in HCC recipients. Out of 113 LT recipients in whom we tried to reach an IS-free status, 17 (15%) received LT for HCC. We achieved a tolerant status in 7 (41.1%) cases and none presented tumour recurrence after a median follow-up of more than 2 years. Despite our study population is still relatively small, we believe that perhaps all LT recipients who had HCC prior to transplant should attempt IS minimization schedules and, whenever possible, complete IS withdrawal as soon as possible after LT. The selection of patients who attempt IS tapering is essential for the good outcome, in particular by the identification of non-invasive transcriptional and/or serological biomarkers of tolerance (9,40).
Furthermore, also “oncological” and “non-oncological” risk factors have to be considered in the selection process of HCC patients who could benefit from IS weaning. The presence of risk factors of tumour recurrence such as tumour size, number of nodules, histological grading, vascular and capsular invasion, pre-transplant alpha-fetoprotein (AFP) plasma levels, close HLA matching between recipient and donor, recipient characteristics (body mass index, age), waiting time to transplantation are in favour of attempting IS withdrawal.
From a practical point of view, potential candidates who could benefit from IS wean should be HCC LT recipients with “oncological” and “non-oncological” risk factors for tumour recurrence, with normal graft function, without autoimmune disease, favourable phenotypic (45) and genomic immunological signature (48) and under low dose of IS drugs early after transplantation. All these patients should undergo liver biopsy before starting IS weaning in order to exclude subclinical rejection. IS tapering should be started 12 months after LT and should be gradually discontinued with the goal of achieving a 50% decrease in drug dosage by month 3 and complete withdrawal by month 6: meanwhile, patients should be followed every 2–3 weeks, with liver function tests, for 3 months and monthly until month 12 after initiation of the weaning process.
Current prospects and future perspectives
Several strategies have been investigated to optimize IS after LT in order to achieve a IS-free status, including: (I) development of target IS regimens (i.e., antibodies or fusion proteins direct against molecules involved in T cell activation or targeting specific immune cell subsets) (49); (II) development of biomarkers to minimize IS (anti-HLA antibodies, non-invasive transcriptional or serological markers of rejection) (50,51); (III) induction of tolerance [e.g., induction of donor-type hematopoietic chimerism in living donor liver transplantation (LDLT)] (52); (IV) modulation of the liver allograft immunogenicity by the use of ex vivo machine perfusion of the liver grafts to modulate inflammatory response (53); (V) generation of bioengineered liver grafts by using acellular liver scaffolds repopulated with patient derived cells (54,55).
All these areas of research are still under evaluation and their applicability in the post-transplant setting are promising. Moreover, researchers are currently exploring the effects of active specific immunotherapy in patients with HCC, which could potentially be used also after LT (24).
In conclusion, new clinical trials are in progress to better clarify the “immunological jungle” present in the post-transplantation setting. Despite data are still limited, the identification of the immunological printing of COT could potentially permit to early select HCC LT recipients who can safely withdraw IS therapy in the early post-transplant period, in the attempt to reduce the risk of tumour recurrence and improve patient and graft outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol 2016;64:433-85. [Crossref] [PubMed]
- Perea Del Pozo E, Bernal Bellido C, Sendín Matín M, et al. Recurrent Hepatocellular Carcinoma After Liver Transplantation: Analysis of Risk Factors. Transplant Proc 2016;48:2990-93. [Crossref] [PubMed]
- Montalvá EM, Cantos M, Boscà A, et al. Prognostic Value of Pre-transplantation Serum Alpha-Fetoprotein Levels in Hepatocellular Carcinoma Recurrence. Transplant Proc 2016;48:2966-8. [Crossref] [PubMed]
- Lai Q, Vitale A, Iesari S, et al. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Orlando G, Manzia T, Baiocchi L, et al. The Tor Vergata weaning off immunosuppression protocol in stable HCV liver transplant patients: the updated follow up at 78 months. Transpl Immunol 2008;20:43-7. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Clavien PA, Muller X, de Oliveira ML, et al. Can immunosuppression be stopped after liver transplantation? Lancet Gastroenterol Hepatol 2017;2:531-7. [Crossref] [PubMed]
- Manzia TM, Angelico R, Baiocchi L, et al. The Tor Vergata weaning of immunosuppression protocols in stable hepatitis C virus liver transplant patients: the 10-year follow-up. Transpl Int 2013;26:259-66. [Crossref] [PubMed]
- Zhou J, Hu Z, Zhang Q, et al. Spectrum of De Novo Cancers and Predictors in Liver Transplantation: Analysis of the Scientific Registry of Transplant Recipients Database. PLoS One 2016;11:e0155179. [Crossref] [PubMed]
- Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol 2013;59:1193-9. [Crossref] [PubMed]
- Bhat M, Sonenberg N, Gores GJ. The mTOR pathway in hepatic malignancies. Hepatology 2013;58:810-8. [Crossref] [PubMed]
- Chinnakotla S, Davis GL, Vasani S, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl 2009;15:1834-42. [Crossref] [PubMed]
- Toso C, Merani S, Bigam DL, et al. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology 2010;51:1237-43. [Crossref] [PubMed]
- Liang W, Wang D, Ling X, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl 2012;18:62-9. [Crossref] [PubMed]
- Cholongitas E, Mamou C, Rodríguez-Castro KI, et al. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int 2014;27:1039-49. [Crossref] [PubMed]
- Geissler EK, Schnitzbauer AA, Zülke C, et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 2016;100:116-25. [Crossref] [PubMed]
- Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer 2008;8:24-36. [Crossref] [PubMed]
- Robson R, Cecka JM, Opelz G, et al. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant 2005;5:2954-60. [Crossref] [PubMed]
- O'Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:1186-91. [Crossref] [PubMed]
- Klintmalm GB, Feng S, Lake JR, et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant 2014;14:1817-27. [Crossref] [PubMed]
- Gomez-Martin C, Bustamante J, Castroagudin JF, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl 2012;18:45-52. [Crossref] [PubMed]
- Aerts M, Benteyn D, Van Vlierberghe H, et al. Current status and perspectives of immune-based therapies for hepatocellular carcinoma. World J Gastroenterol 2016;22:253-61. [Crossref] [PubMed]
- Ashton-Chess J, Giral M, Brouard S, et al. Spontaneous operational tolerance after immunosuppressive drug withdrawal in clinical renal allotransplantation. Transplantation 2007;84:1215-9. [Crossref] [PubMed]
- Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant 2006;6:1774-80. [Crossref] [PubMed]
- Sumimoto R, Kamada N. Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation 1990;50:678-82. [Crossref] [PubMed]
- Qian S, Demetris AJ, Murase N, et al. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology 1994;19:916-24. [Crossref] [PubMed]
- Sriwatanawongsa V, Davies HS, Calne RY. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nat Med 1995;1:428-32. [Crossref] [PubMed]
- Reyes J, Zeevi A, Ramos H, et al. Frequent achievement of a drug-free state after orthotopic liver transplantation. Transplant Proc 1993;25:3315-9. [PubMed]
- Mazariegos GV, Reyes J, Marino I, et al. Risks and benefits of weaning immunosuppression in liver transplant recipients: long-term follow-up. Transplant Proc 1997;29:1174-7. [Crossref] [PubMed]
- Girlanda R, Rela M, Williams R, et al. Long-term outcome of immunosuppression withdrawal after liver transplantation. Transplant Proc 2005;37:1708-9. [Crossref] [PubMed]
- Tisone G, Orlando G, Cardillo A, et al. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol 2006;44:702-9. [Crossref] [PubMed]
- Benítez C, Londoño MC, Miquel R, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 2013;58:1824-35. [Crossref] [PubMed]
- Pons JA, Revilla-Nuin B, Baroja-Mazo A, et al. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplantation 2008;86:1370-8. [Crossref] [PubMed]
- Tryphonopoulos P, Ruiz P, Weppler D, et al. Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation 2010;90:1556-61. [Crossref] [PubMed]
- Feng S, Ekong UD, Lobritto SJ, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA 2012;307:283-93. [Crossref] [PubMed]
- de la Garza RG, Sarobe P, Merino J, et al. Trial of complete weaning from immunosuppression for liver transplant recipients: factors predictive of tolerance. Liver Transpl 2013;19:937-44. [Crossref] [PubMed]
- Bohne F, Londoño MC, Benítez C, et al. HCV-induced immune responses influence the development of operational tolerance after liver transplantation in humans. Sci Transl Med 2014;6:242ra81. [Crossref] [PubMed]
- Adams DH, Sanchez-Fueyo A, Samuel D. From immunosuppression to tolerance. J Hepatol 2015;62:S170-85. [Crossref] [PubMed]
- Sánchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology 2011;140:51-64. [Crossref] [PubMed]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89-95. [Crossref] [PubMed]
- Mazariegos GV, Zahorchak AF, Reyes J, et al. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant 2003;3:689-96. [Crossref] [PubMed]
- Li Y, Koshiba T, Yoshizawa A, et al. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am J Transplant 2004;4:2118-25. [Crossref] [PubMed]
- Martínez-Llordella M, Puig-Pey I, Orlando G, et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant 2007;7:309-19. [Crossref] [PubMed]
- Donckier V, Troisi R, Toungouz M, et al. Donor stem cell infusion after non-myeloablative conditioning for tolerance induction to HLA mismatched adult living-donor liver graft. Transpl Immunol 2004;13:139-46. [Crossref] [PubMed]
- Giakoustidis AE, Giakoustidis DE. Immunosuppression strategies in liver transplantation patient; patients with hepatocellular carcinoma. Immunotherapy 2017;9:197-206. [Crossref] [PubMed]
- Bohne F, Martínez-Llordella M, Lozano JJ, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest 2012;122:368-82. [Crossref] [PubMed]
- Page EK, Dar WA, Knechtle SJ. Biologics in organ transplantation. Transpl Int 2012;25:707-19. [Crossref] [PubMed]
- Eason JD, Cohen AJ, Nair S, et al. Tolerance: is it worth the risk? Transplantation 2005;79:1157-9. [Crossref] [PubMed]
- Schlickeiser S, Boës D, Streitz M, et al. The use of novel diagnostics to individualize immunosuppression following transplantation. Transpl Int 2015;28:911-20. [Crossref] [PubMed]
- Kawai T, Sachs DH. Tolerance induction: hematopoietic chimerism. Curr Opin Organ Transplant 2013;18:402-7. [PubMed]
- Schlegel A, Kron P, Graf R, et al. Hypothermic Oxygenated Perfusion (HOPE) downregulates the immune response in a rat model of liver transplantation. Ann Surg 2014;260:931-7; discussion 937-8. [Crossref] [PubMed]
- Moser PT, Ott HC. Recellularization of organs: what is the future for solid organ transplantation? Curr Opin Organ Transplant 2014;19:603-9. [Crossref] [PubMed]
- Hata T, Uemoto S, Kobayashi E. Transplantable liver production plan: "Yamaton"--liver project, Japan. Organogenesis 2013;9:235-8. [Crossref] [PubMed]
Cite this article as: Angelico R, Parente A, Manzia TM. Using a weaning immunosuppression protocol in liver transplantation recipients with hepatocellular carcinoma: a compromise between the risk of recurrence and the risk of rejection? Transl Gastroenterol Hepatol 2017;2:74.


