Hemocoagulase might not control but worsen gastrointestinal bleeding in an elderly patient with type II respiratory failure
Introduction
Gastrointestinal bleeding is a lethal emergency condition, especially in elderly patients (1-3). Emergent gastrointestinal endoscopy is necessary to uncover the source and etiology of bleeding as soon as possible and to guide the subsequent therapeutic strategy (4,5). However, in very sick patients, endoscopy is potentially contraindicated. In this setting, conventional treatment options include life support, blood transfusion, somatostatin, and anti-acid drugs (6,7).
Hemocoagulase is a snake venom based mixture of thrombin-like and thromboplastin-like enzymes. Many different products are available, with different characteristics according to the source of the enzyme purified. Major mechanism of haemostasis by hemocoagulase(s) is to cleave fibrinogen by release of fibrinopeptide -A or both fibrinopeptide-A and -B, and then form fibrin monomer; moreover, it activates factor Xa and helps in the formation of thrombin at the site of hemorrhage. It is also found to stabilize the fibrin by an action on the factor XIIIa (8-13). In China, it has been widely used for the management of bleeding in patients undergoing surgery, including breast cancer surgery (14), fracture-related hip hemiarthroplasty (15), spinal fusion surgery (16,17), dental extraction (18), and abdominal surgery (19). Several studies also suggested that hemocoagulase might be a choice for the treatment of gastrointestinal bleeding. An early pilot study showed that local spray of reptilase was effective for controlling bleeding from gastric or duodenal ulcers and gastric cancer (20). Recently, a randomized controlled trial has demonstrated that topical hemocoagulase spray during the endoscopic submucosal dissection and esophageal tunneling techniques procedures might significantly reduce the incidence of late bleeding and overall complications, but not the rate of successful haemostasis (21). However, the potential adverse events related to hemocoagulase are poorly recognized. Venom-induced consumption coagulopathy is one of the commonest types of coagulopathy, which results from snakebite and occurs in envenoming due to several snakes, so that caution should be warranted in using such products obtained by snake venom procoagulant toxins (22).
Herein, we reported an elderly patient type II respiratory failure in whom hemocoagulase might not control but worsen gastrointestinal bleeding secondary to hypofibrinogenemia.
Case presentation
On June 2, 2017, a 79-year-old male was admitted to his local hospital due to pneumonia and type II respiratory failure and was treated with antibiotics, bronchodilators drugs, and mechanical ventilation. He had chronic bronchitis for 50 years, diabetes for 20 years, and arterial hypertension for 5 years. He denied any history of liver or hematological diseases. However, he received aspirin for 2 years. On June 7, 2017, he developed intermittent melena without haematemesis during his hospitalization. A diagnosis of gastrointestinal bleeding secondary to stress was considered. Proton pump inhibitors (i.e., lansoprazole and pantoprazole), somatostatin, and hemocoagulase for injection 10 units were prescribed. Hemocoagulase used in this case was extracted from the venom of Chinese Agkistrodon blomhoffii ussurensis living in Changbai Mountain. However, bleeding continued.
On June 15, 2017, he was transferred to our hospital. At the department of emergency medicine, laboratory tests demonstrated that red blood cell was 1.89×1012/L (reference range: 4.3–5.8×1012/L), hemoglobin concentration was 59 g/L (reference range: 130–175 g/L), hematocrit was 18.4% (reference range: 40–50%), platelets count was 175×109/L (reference range: 125–350×109/L), prothrombin time was 27.7 s (reference range: 11.5–14.5 s), international normalized ratio was 2.5, activated partial thromboplastin time was 45.4 s (reference range: 28–40 s), fibrinogen was 0.60 g/L (reference range: 2.00–4.00 g/L), d-dimer was 4.34 mg/L (reference range: 0.01–0.55 mg/L), and albumin was 26.8 g/L (reference range: 40–55 g/L). Chest and abdominal computed tomography scans demonstrated moderate pleural effusion and mild ascites and pericardial effusion. No remarkable lesion was found in the gastrointestinal tract. Proton pump inhibitor (i.e., esomeprazole), somatostatin, and hemocoagulase for injection 2 units were given. Additionally, red blood cell 6 units and common frozen plasma 400 mL were infused.
On June 16, 2017, he was admitted to our department. Melena continued. Fresh blood remained drained via a nasogastric tube. Heart rate was 110 bpm and blood pressure was 150/80 mmHg. Blood gas analysis demonstrated that pH was 7.38 (reference range: 7.35–7.45), pCO2 was 57 mmHg (reference range: 35–45 mmHg), pO2 was 56 mmHg (reference range: 80–100 mmHg), HCO3- was 33.7 mmol/L (reference range: 22–26 mmol/L), and buffer excess (BE) was 8.6 mmol/L (reference range: −2 to 3 mmol/L). Thus, a diagnosis of respiratory failure was considered. A respiratory consultation was performed. Non-invasive ventilation, ambroxol, aminophylline, and ipratropium bromide were prescribed. Respiratory failure was not further aggravated. Additionally, laboratory tests were repeated, showing that red blood cell was 2.52×1012/L, hemoglobin concentration was 74 g/L, hematocrit was 23.2%, platelets count was 143×109/L, prothrombin time was 29.7 s, international normalized ratio was 2.73, activated partial thromboplastin time was 41.3 s, fibrinogen was 0.65 g/L, d-dimer was 2.27 mg/L, and albumin was 26.8 g/L. At that time, disseminated intravascular coagulation was highly suspected. A hematological consultation was performed. Hematologists suspected that coagulation disorders might be associated with the use of hemocoagulase, because this drug could consume fibrinogen and further activate the coagulation system, possibly producing pathological intravascular coagulation. Thus, hemocoagulase had been never given at our department. During his hospitalization, red blood cell 11.6 units, fresh frozen plasma 810 mL, cryoprecipitate 27.5 units, human fibrinogen 2 g, and human albumin 120 g were given.
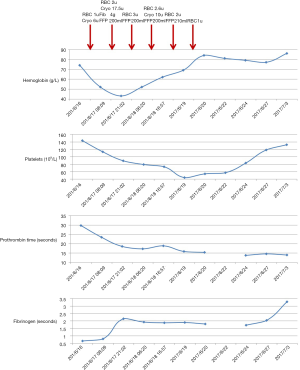
Since June 24, 2017, stool occult blood test became negative. Coagulation disorders had been gradually corrected (Figure 1). On July 3, 2017, blood gas analysis demonstrated that pH was 7.44, pCO2 was 53 mmHg, pO2 was 48 mmHg, HCO3- was 36.0 mmol/L, and BE was 11.8 mmol/L. Laboratory tests demonstrated that red blood cell was 2.77×1012/L, hemoglobin concentration was 86 g/L, hematocrit was 26.7%, platelets count was 132×109/L, prothrombin time was 13.9 s, international normalized ratio was 1.06, activated partial thromboplastin time was 42.8 s, fibrinogen was 3.28 g/L, d-dimer was 3.65 mg/L, and albumin was 31.9 g/L. Considering that his respiratory failure was not completely corrected, his relatives refused any gastrointestinal endoscopy. And then, he was discharged.
Discussion
In spite of its benefit in controlling bleeding (23), a major drawback of hemocoagulase is the reduction of fibrinogen levels (i.e., hypofibrinogenemia). Indeed, Wei et al. found that larger doses of hemocoagulase might be associated with postoperative fibrinogen deficiency in patients treated with surgical removal of intracranial tumors, which increased the need for blood transfusion (24). Zhou also reported that 7 cases receiving intravenous hemocoagulase developed hypofibrinogenemia after endoscopic excisions of colon polyps (25). Notably, none of 13 patients who did not receive intravenous hemocoagulase developed hypofibrinogenemia during the same period. Wang et al. reported that fibrinogen levels significantly decreased in 3 patients after hemocoagulase administration for 4–5 days and then 2 of them developed persistent bleeding (26). Additionally, Zhang et al. reported that a case received hemocoagulase before and after endoscopic sinus surgery and developed hypofibrinogenemia and its related nasal bleeding (27). Similarly, in our case, gastrointestinal bleeding persisted and worsened after the use of hemocoagulase. This unexpected phenomenon might be explained by hypofibrinogenemia secondary to use of hemocoagulase. Based on these experiences, we should pay more attention to the use of hemocoagulase. When to start and stop hemocoagulase should be further assessed.
Although previous studies suggest the hemostatic effect of hemocoagulase for the management of gastrointestinal bleeding, local spray of hemocoagulase during endoscopic procedures, rather than systemic administration of hemocoagulase, is performed. Thus, intravenous injection of hemocoagulase in patients with gastrointestinal bleeding remains questioned and the possibility of disseminated intravascular coagulation should be considered in the risk-benefit balance.
According to its instruction, hemocoagulase can be completely cleared after 3–4 days. However, hemocoagulase-induced hypofibrinogenemia is potentially lethal before the elimination of this drug. Supplementation of cryoprecipitate and infusion of human fibrinogen should be life-saving. In the case of shortage of blood products, the patient’s outcome may be dismal.
In conclusion, our case report suggests that intravenous administration of hemocoagulase should be cautiously used for the management of gastrointestinal bleeding, because it might increase the risk of hypofibrinogenemia and its related bleeding. Additionally, we should closely monitor the fibrinogen levels and bleeding risk after the use of hemocoagulase.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Rockall TA, Logan RF, Devlin HB, et al. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 1995;311:222-6. [Crossref] [PubMed]
- Hearnshaw SA, Logan RF, Lowe D, et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011;60:1327-35. [Crossref] [PubMed]
- Prasad Kerlin M, Tokar JL. Acute gastrointestinal bleeding. Ann Intern Med 2013;159:ITC2-1, ITC2-2, ITC2-3, ITC2-4, ITC2-5, ITC2-6, ITC2-7, ITC2-8, ITC2-9, ITC2-10, ITC2-11, ITC2-12, ITC2-13, ITC2-14, ITC2-15; quiz ITC2-16.
- Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:a1-46. [Crossref] [PubMed]
- Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012;107:345-60. [Crossref] [PubMed]
- Lau JY, Barkun A, Fan DM, et al. Challenges in the management of acute peptic ulcer bleeding. Lancet 2013;381:2033-43. [Crossref] [PubMed]
- Sung JJ, Chan FK, Chen M, et al. Asia-Pacific Working Group consensus on non-variceal upper gastrointestinal bleeding. Gut 2011;60:1170-7. [Crossref] [PubMed]
- Bell WR Jr. Defibrinogenating enzymes. Drugs 1997;54 Suppl 3:18-30; discussion 30-1. [Crossref] [PubMed]
- Gornitskaia OV, Platonova TN, Volkov GL. Enzymes of snake venoms. Ukr Biokhim Zh (1999) 2003.22-32. [PubMed]
- Castro HC, Zingali RB, Albuquerque MG, et al. Snake venom thrombin-like enzymes: from reptilase to now. Cell Mol Life Sci 2004;61:843-56. [Crossref] [PubMed]
- Sakai J, Zhang S, Chen H, et al. Primary structure of a thrombin-like serine protease, kangshuanmei, from the venom of Agkistrodon halys brevicaudus stejneger. Toxicon 2006;48:313-22. [Crossref] [PubMed]
- You WK, Choi WS, Koh YS, et al. Functional characterization of recombinant batroxobin, a snake venom thrombin-like enzyme, expressed from Pichia pastoris. FEBS Lett 2004;571:67-73. [Crossref] [PubMed]
- Lü HM, Li CL, Dong JC, et al. Hemostatic effect of hemocoagulase agkistrodon and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2008;16:883-5. [PubMed]
- Lu X, Yang X, Zhu M, et al. Hemostatic Effect of Hemocoagulase Agkistrodon on Surgical Wound in Breast Cancer Surgery. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2017;39:183-7. [PubMed]
- Qiu M, Zhang X, Cai H, et al. The impact of hemocoagulase for improvement of coagulation and reduction of bleeding in fracture-related hip hemiarthroplasty geriatric patients: A prospective, single-blinded, randomized, controlled study. Injury 2017;48:914-9. [Crossref] [PubMed]
- Mn R, Shetty AP, Dumpa SR, et al. Effectiveness and Safety of Batroxobin, Tranexamic acid and a Combination in reduction of blood loss in Lumbar Spinal Fusion Surgery. Spine (Phila Pa 1976) 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Hu HM, Chen L, Frary CE, et al. The beneficial effect of Batroxobin on blood loss reduction in spinal fusion surgery: a prospective, randomized, double-blind, placebo-controlled study. Arch Orthop Trauma Surg 2015;135:491-7. [Crossref] [PubMed]
- Joshi SA, Gadre KS, Halli R, et al. Topical use of Hemocoagulase (Reptilase): A simple and effective way of managing post-extraction bleeding. Ann Maxillofac Surg 2014;4:119. [Crossref] [PubMed]
- Wei JM, Zhu MW, Zhang ZT, et al. A multicenter, phase III trial of hemocoagulase Agkistrodon: hemostasis, coagulation, and safety in patients undergoing abdominal surgery. Chin Med J (Engl) 2010;123:589-93. [PubMed]
- Liu B, Gong B, Wang H. Clinical application of endoscopic injection of medication for stopping gastric bleeding. Zhonghua Nei Ke Za Zhi 1995;34:446-8. [PubMed]
- Wang T, Wang DN, Liu WT, et al. Hemostatic effect of topical hemocoagulase spray in digestive endoscopy. World J Gastroenterol 2016;22:5831-6. [Crossref] [PubMed]
- Maduwage K, Isbister GK. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl Trop Dis 2014;8:e3220. [Crossref] [PubMed]
- Seon GM, Lee MH, Kwon BJ, et al. Functional improvement of hemostatic dressing by addition of recombinant batroxobin. Acta Biomater 2017;48:175-85. [Crossref] [PubMed]
- Wei N, Jia Y, Wang X, et al. Risk Factors for Postoperative Fibrinogen Deficiency after Surgical Removal of Intracranial Tumors. PLoS One 2015;10:e0144551. [Crossref] [PubMed]
- Zhou HB. Hypofibrinogenemia Caused by Hemocoagulase After Colon Polyps Excision. Am J Case Rep 2017;18:291-3. [Crossref] [PubMed]
- Wang Z, Li J, Cao L, et al. Zhonghua Xue Ye Xue Za Zhi 2014;35:50-2. [Hypofibrinogenemia caused by long-term administration of hemocoagulase: three cases report and literature review]. [PubMed]
- Zhang C, Liu Y, Liu G. Hypofibrinogenemia caused by hemocoagulase after endoscopic sinus surgery: a case report. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2016;30:70-1. [PubMed]
Cite this article as: Qi X, Wang J, Yu X, De Stefano V, Li H, Wu C, Zeng Q, Zhang Y, Ren L, Lin H, Deng J, Guo X. Hemocoagulase might not control but worsen gastrointestinal bleeding in an elderly patient with type II respiratory failure. Transl Gastroenterol Hepatol 2017;2:71.


